The rabies virus glycoprotein receptor p75NTR is not essential for rabies virus infection
- PMID: 17928338
- PMCID: PMC2168826
- DOI: 10.1128/JVI.02368-06
The rabies virus glycoprotein receptor p75NTR is not essential for rabies virus infection
Abstract
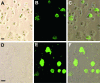
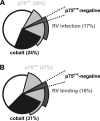
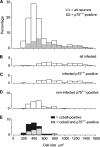
Rabies virus glycoprotein (RVG) is known to be the only factor that mediates rabies infection. The neurotrophin receptor (p75(NTR)), through its cysteine-rich domain 1, is a specific receptor for RVG and neutralizes virus infectivity, but its role in virus infection has remained obscure. We used adult mouse dorsal root ganglion (DRG) neurons as a model to study the role of p75(NTR) in RV infection of primary neurons. We show that RV infects around 20% of DRG neurons, of which more than 80% are p75(NTR) positive, have large diameters, and are capsaicin insensitive. Surprisingly, RV binding and infection are absent in about half of the p75(NTR)-expressing DRG neurons which have small diameters and are often capsaicin sensitive. This indicates that p75(NTR) is not sufficient to mediate RV interaction in sensory neurons. The rate and specificity of neural infection are unchanged in RV-infected p75(NTRExonIV-/-) mice that lack all extracellular receptor domains and in wild-type mice infected with two independent RV mutants that lack p75(NTR) binding. Accordingly, the mortality rate is unchanged in the absence of RV-p75(NTR) interaction. We conclude that although p75(NTR) is a receptor for soluble RVG in transfected cells of heterologous expression systems, an RVG-p75(NTR) interaction is not necessary for RV infection of primary neurons. This means that other receptors are required to mediate RV infection in vivo and in vitro.
Figures





Similar articles
-
Mutations conferring resistance to neutralization by a soluble form of the neurotrophin receptor (p75NTR) map outside of the known antigenic sites of the rabies virus glycoprotein.J Virol. 2002 Nov;76(21):10756-65. doi: 10.1128/jvi.76.21.10756-10765.2002. J Virol. 2002. PMID: 12368318 Free PMC article.
-
Rabies virus glycoprotein (RVG) is a trimeric ligand for the N-terminal cysteine-rich domain of the mammalian p75 neurotrophin receptor.J Biol Chem. 2002 Oct 4;277(40):37655-62. doi: 10.1074/jbc.M201374200. Epub 2002 Aug 5. J Biol Chem. 2002. PMID: 12163480
-
Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus.EMBO J. 1998 Dec 15;17(24):7250-9. doi: 10.1093/emboj/17.24.7250. EMBO J. 1998. PMID: 9857182 Free PMC article.
-
Rabies virus receptors.J Neurovirol. 2005 Feb;11(1):82-7. doi: 10.1080/13550280590900427. J Neurovirol. 2005. PMID: 15804965 Review.
-
Pathogenesis of rabies.Curr Top Microbiol Immunol. 2005;292:45-56. doi: 10.1007/3-540-27485-5_3. Curr Top Microbiol Immunol. 2005. PMID: 15981467 Review.
Cited by
-
Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery.PLoS Pathog. 2014 Aug 28;10(8):e1004348. doi: 10.1371/journal.ppat.1004348. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25165859 Free PMC article.
-
Application of Recombinant Rabies Virus to Xenopus Tadpole Brain.eNeuro. 2021 Jun 7;8(4):ENEURO.0477-20.2021. doi: 10.1523/ENEURO.0477-20.2021. Online ahead of print. eNeuro. 2021. PMID: 34099488 Free PMC article.
-
Transferrin Receptor Protein 1 Cooperates with mGluR2 To Mediate the Internalization of Rabies Virus and SARS-CoV-2.J Virol. 2023 Feb 28;97(2):e0161122. doi: 10.1128/jvi.01611-22. Epub 2023 Feb 13. J Virol. 2023. PMID: 36779763 Free PMC article.
-
Pseudotyping Lentiviral Vectors: When the Clothes Make the Virus.Viruses. 2020 Nov 16;12(11):1311. doi: 10.3390/v12111311. Viruses. 2020. PMID: 33207797 Free PMC article. Review.
-
Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons.J Virol. 2010 May;84(9):4697-705. doi: 10.1128/JVI.02654-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181692 Free PMC article.
References
-
- Baldwin, A. N., and E. M. Shooter. 1995. Zone mapping of the binding domain of the rat low affinity nerve growth factor receptor by the introduction of novel N-glycosylation sites. J. Biol. Chem. 270:4594-4602. - PubMed
-
- Bergmann, I., J. V. Priestley, S. B. McMahon, E. B. Brocker, K. V. Toyka, and M. Koltzenburg. 1997. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur. J. Neurosci. 9:18-28. - PubMed
-
- Castellanos, J. E., D. R. Castaneda, A. E. Velandia, and H. Hurtado. 1997. Partial inhibition of the in vitro infection of adult mouse dorsal root ganglion neurons by rabies virus using nicotinic antagonists. Neurosci. Lett. 229:198-200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

