A protective and broadly cross-neutralizing epitope of human papillomavirus L2
- PMID: 17928339
- PMCID: PMC2168823
- DOI: 10.1128/JVI.00936-07
A protective and broadly cross-neutralizing epitope of human papillomavirus L2
Abstract
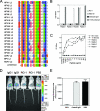
We generated a monoclonal antibody, RG-1, that binds to highly conserved L2 residues 17 to 36 and neutralizes human papillomavirus 16 (HPV16) and HPV18. Passive immunotherapy with RG-1 was protective in mice. Antiserum to the HPV16 L2 peptide comprising residues 17 to 36 (peptide 17-36) neutralized pseudoviruses HPV5, HPV6, HPV16, HPV 18, HPV31, HPV 45, HPV 52, HPV 58, bovine papillomavirus 1, and HPV11 native virions. Depletion of HPV16 L2 peptide 17-36-reactive antibodies from cross-neutralizing rabbit and human L2-specific sera abolished cross-neutralization and drastically reduced neutralization of the cognate type. This cross-neutralization of diverse HPVs associated with cervical cancer, genital warts, and epidermodysplasia verruciformis suggests the possibility of a broadly protective, peptide-based vaccine.
Figures

References
-
- Campo, M. S. 1997. Vaccination against papillomavirus in cattle. Clin. Dermatol. 15:275-283. - PubMed
-
- Chandrachud, L. M., G. J. Grindlay, G. M. McGarvie, B. W. O'Neil, E. R. Wagner, W. F. Jarrett, and M. S. Campo. 1995. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 211:204-208. - PubMed
-
- Christensen, N. D., J. W. Kreider, N. C. Kan, and S. L. DiAngelo. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572-579. - PubMed
-
- Embers, M. E., L. R. Budgeon, T. D. Culp, C. A. Reed, M. D. Pickel, and N. D. Christensen. 2004. Differential antibody responses to a distinct region of human papillomavirus minor capsid proteins. Vaccine 22:670-680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

