Double-labeled rabies virus: live tracking of enveloped virus transport
- PMID: 17928343
- PMCID: PMC2224359
- DOI: 10.1128/JVI.01342-07
Double-labeled rabies virus: live tracking of enveloped virus transport
Abstract
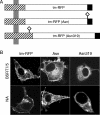
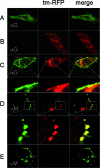
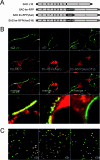
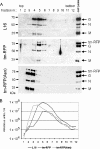
Here we describe a strategy to fluorescently label the envelope of rabies virus (RV), of the Rhabdoviridae family, in order to track the transport of single enveloped viruses in living cells. Red fluorescent proteins (tm-RFP) were engineered to comprise the N-terminal signal sequence and C-terminal transmembrane spanning and cytoplasmic domain sequences of the RV glycoprotein (G). Two variants of tm-RFP were transported to and anchored in the cell surface membrane, independent of glycosylation. As shown by confocal microscopy, tm-RFP colocalized at the cell surface with the RV matrix and G protein and was incorporated into G gene-deficient virus particles. Recombinant RV expressing the membrane-anchored tm-RFP in addition to G yielded infectious viruses with mosaic envelopes containing both tm-RFP and G. Viable double-labeled virus particles comprising a red fluorescent envelope and a green fluorescent ribonucleoprotein were generated by expressing in addition an enhanced green fluorescent protein-phosphoprotein fusion construct (S. Finke, K. Brzozka, and K. K. Conzelmann, J. Virol. 78:12333-12343, 2004). Individual enveloped virus particles were observed under live cell conditions as extracellular particles and inside endosomal vesicles. Importantly, double-labeled RVs were transported in the retrograde direction over long distances in neurites of in vitro-differentiated NS20Y neuroblastoma cells. This indicates that the typical retrograde axonal transport of RV to the central nervous system involves neuronal transport vesicles in which complete enveloped RV particles are carried as a cargo.
Figures


Similar articles
-
Anterograde glycoprotein-dependent transport of newly generated rabies virus in dorsal root ganglion neurons.J Virol. 2014 Dec;88(24):14172-83. doi: 10.1128/JVI.02254-14. Epub 2014 Oct 1. J Virol. 2014. PMID: 25275124 Free PMC article.
-
Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3.J Virol. 2005 Jun;79(12):7673-81. doi: 10.1128/JVI.79.12.7673-7681.2005. J Virol. 2005. PMID: 15919920 Free PMC article.
-
Pseudotyping of G-Gene-Deficient Rabies Virus.Cold Spring Harb Protoc. 2015 Dec 2;2015(12):pdb.prot089417. doi: 10.1101/pdb.prot089417. Cold Spring Harb Protoc. 2015. PMID: 26631128
-
Pathogenesis of rabies.Curr Top Microbiol Immunol. 2005;292:45-56. doi: 10.1007/3-540-27485-5_3. Curr Top Microbiol Immunol. 2005. PMID: 15981467 Review.
-
Replication strategies of rabies virus.Virus Res. 2005 Aug;111(2):120-31. doi: 10.1016/j.virusres.2005.04.004. Virus Res. 2005. PMID: 15885837 Review.
Cited by
-
Rabies Internalizes into Primary Peripheral Neurons via Clathrin Coated Pits and Requires Fusion at the Cell Body.PLoS Pathog. 2016 Jul 27;12(7):e1005753. doi: 10.1371/journal.ppat.1005753. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27463226 Free PMC article.
-
Real-time Imaging of Rabies Virus Entry into Living Vero cells.Sci Rep. 2015 Jul 7;5:11753. doi: 10.1038/srep11753. Sci Rep. 2015. PMID: 26148807 Free PMC article.
-
Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons.PLoS Pathog. 2018 Jul 20;14(7):e1007188. doi: 10.1371/journal.ppat.1007188. eCollection 2018 Jul. PLoS Pathog. 2018. PMID: 30028873 Free PMC article.
-
Limited brain metabolism changes differentiate between the progression and clearance of rabies virus.PLoS One. 2014 Apr 24;9(4):e87180. doi: 10.1371/journal.pone.0087180. eCollection 2014. PLoS One. 2014. PMID: 24763072 Free PMC article.
-
An mRNA Vaccine Encoding Rabies Virus Glycoprotein Induces Protection against Lethal Infection in Mice and Correlates of Protection in Adult and Newborn Pigs.PLoS Negl Trop Dis. 2016 Jun 23;10(6):e0004746. doi: 10.1371/journal.pntd.0004746. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27336830 Free PMC article.
References
-
- Dalton, K. P., and J. K. Rose. 2001. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology 279414-421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

