The U95 protein of human herpesvirus 6B interacts with human GRIM-19: silencing of U95 expression reduces viral load and abrogates loss of mitochondrial membrane potential
- PMID: 17928352
- PMCID: PMC2224593
- DOI: 10.1128/JVI.01156-07
The U95 protein of human herpesvirus 6B interacts with human GRIM-19: silencing of U95 expression reduces viral load and abrogates loss of mitochondrial membrane potential
Abstract
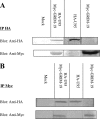
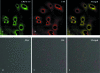
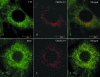
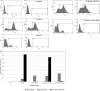
To better understand the pathogenesis of human herpesvirus 6 (HHV-6), it is important to elucidate the functional aspects of immediate-early (IE) genes at the initial phase of the infection. To study the functional role of the HHV-6B IE gene encoding U95, we generated a U95-Myc fusion protein and screened a pretransformed bone marrow cDNA library for U95-interacting proteins, using yeast-two hybrid analysis. The most frequently appearing U95-interacting protein identified was GRIM-19, which belongs to the family of genes associated with retinoid-interferon mortality and serves as an essential component of the oxidative phosphorylation system. This interaction was verified by both coimmunoprecipitation and confocal microscopic coimmunolocalization. Short-term HHV-6B infection of MT-4 T-lymphocytic cells induced syncytial formation, resulted in decreased mitochondrial membrane potential, and led to progressively pronounced ultrastructural changes, such as mitochondrial swelling, myelin-like figures, and a loss of cristae. Compared to controls, RNA interference against U95 effectively reduced the U95 mRNA copy number and abrogated the loss of mitochondrial membrane potential. Our results indicate that the high affinity between U95 early viral protein and GRIM-19 may be closely linked to the detrimental effect of HHV-6B infection on mitochondria. These findings may explain the alternative cell death mechanism of expiration, as opposed to apoptosis, observed in certain productively HHV-6B-infected cells. The interaction between U95 and GRIM-19 is thus functionally and metabolically significant in HHV-6B-infected cells and may be a means through which HHV-6B modulates cell death signals by interferon and retinoic acid.
Figures


Similar articles
-
Cooperative activation of the human herpesvirus 6B U79/80 early gene promoter by immediate-early proteins IE1B and IE2B.Microbiol Immunol. 2020 Nov;64(11):747-761. doi: 10.1111/1348-0421.12844. Epub 2020 Oct 16. Microbiol Immunol. 2020. PMID: 32910457
-
Genome-Wide Approach to the CD4 T-Cell Response to Human Herpesvirus 6B.J Virol. 2019 Jun 28;93(14):e00321-19. doi: 10.1128/JVI.00321-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043533 Free PMC article.
-
GRIM-19 associates with the serine protease HtrA2 for promoting cell death.Oncogene. 2007 Jul 19;26(33):4842-9. doi: 10.1038/sj.onc.1210287. Epub 2007 Feb 12. Oncogene. 2007. PMID: 17297443
-
Molecular biology of human herpesviruses 6A and 6B.Infect Agents Dis. 1993 Dec;2(6):343-60. Infect Agents Dis. 1993. PMID: 8012736 Review.
-
Latency, Integration, and Reactivation of Human Herpesvirus-6.Viruses. 2017 Jul 24;9(7):194. doi: 10.3390/v9070194. Viruses. 2017. PMID: 28737715 Free PMC article. Review.
Cited by
-
Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).J Transl Med. 2018 Oct 1;16(1):268. doi: 10.1186/s12967-018-1644-y. J Transl Med. 2018. PMID: 30285773 Free PMC article. Review.
-
Salivary DNA Loads for Human Herpesviruses 6 and 7 Are Correlated With Disease Phenotype in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Front Med (Lausanne). 2021 Aug 6;8:656692. doi: 10.3389/fmed.2021.656692. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34422848 Free PMC article.
-
GRIM-19 disrupts E6/E6AP complex to rescue p53 and induce apoptosis in cervical cancers.PLoS One. 2011;6(7):e22065. doi: 10.1371/journal.pone.0022065. Epub 2011 Jul 12. PLoS One. 2011. PMID: 21765936 Free PMC article.
-
Immunomodulation and immunosuppression by human herpesvirus 6A and 6B.Future Virol. 2013 Mar;8(3):273-287. doi: 10.2217/fvl.13.7. Future Virol. 2013. PMID: 24163703 Free PMC article.
-
Complete Genome Sequence of Germline Chromosomally Integrated Human Herpesvirus 6A and Analyses Integration Sites Define a New Human Endogenous Virus with Potential to Reactivate as an Emerging Infection.Viruses. 2016 Jan 15;8(1):19. doi: 10.3390/v8010019. Viruses. 2016. PMID: 26784220 Free PMC article.
References
-
- Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184545-552. - PubMed
-
- Andrews, H. E., P. P. Nichols, D. Bates, and D. M. Turnbull. 2005. Mitochondrial dysfunction plays a key role in progressive axonal loss in multiple sclerosis. Med. Hypotheses 64669-677. - PubMed
-
- Angell, J. E., D. J. Lindner, P. S. Shapiro, E. R. Hofmann, and D. V. Kalvakolanu. 2000. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J. Biol. Chem. 27533416-33426. - PubMed
-
- Arvin, A., G. Campadielli-Fiume, E. Mocarski, P. S. Moore, B. Roizman, R. Whitley, and K. Yamanishi (ed.). 2007. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. - PubMed
-
- Ashshi, A. M., R. J. Cooper, P. E. Klapper, O. Al-Jiffri, and L. Moore. 2000. Detection of human herpes virus 6 DNA in fetal hydrops. Lancet 3551519-1520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

