Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro
- PMID: 17928355
- PMCID: PMC2168870
- DOI: 10.1128/JVI.01568-07
Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro
Abstract
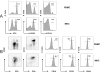
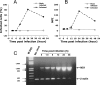
Understanding the pathogenesis of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) requires the precise identification of dengue virus (DV)-permissive target cells. In a previous study using unfractionated human peripheral blood mononuclear cells, we found that monocytes, but not B or T cells, were the principal DV-permissive cells in the absence of DV-immune pooled human sera (PHS) and the major mediators of antibody-dependent enhancement in the presence of PHS. To further identify DV-permissive target cells in other tissues and organs, we isolated human splenic mononuclear cells (MNCs), inoculated them with DV type 2 (strain 16681) in the presence or absence of PHS, and assessed their infection either directly using flow cytometry and reverse transcription-PCR (RT-PCR) assays or indirectly by plaque assay. We found that in the absence of PHS, a small proportion of splenic macrophages appeared to be positive for DV antigens in comparison to staining controls by the flow cytometric assay (0.77% +/- 1.00% versus 0.18% +/- 0.12%; P = 0.07) and that viral RNA was detectable by the RT-PCR assay in MNCs exposed to DV. Additionally, supernatants from cultures of DV-exposed MNCs contained infectious virions that were readily detectable by plaque assay. The magnitude of infection was significantly enhanced in splenic macrophages in the presence of highly diluted PHS (5.41% +/- 3.53% versus 0.77% +/- 1.00%; P = 0.001). In contrast, primary T and B cells were not infected in either the presence or absence of PHS. These results provide evidence, for the first time, that human primary splenic macrophages, rather than B or T cells, are the principal DV-permissive cells in the spleen and that they may be uniquely important in the initial steps of immune enhancement that leads to DHF/DSS in some DV-infected individuals.
Figures
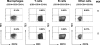
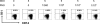




References
-
- Avirutnan, P., P. Malasit, B. Seliger, S. Bhakdi, and M. Husmann. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 161:6338-6346. - PubMed
-
- Gordon, S., and P. R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953-964. - PubMed
-
- Green, S., and A. Rothman. 2006. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19:429-436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

