Antibody detection and kinetics of antibody production during early stages of immunization with hepatitis B virus vaccine
- PMID: 17928429
- PMCID: PMC2168390
- DOI: 10.1128/CVI.00158-07
Antibody detection and kinetics of antibody production during early stages of immunization with hepatitis B virus vaccine
Abstract
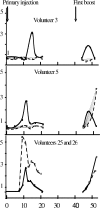
Antibodies to influenza virus and human immunodeficiency virus are detectable in B cells during the early stages of the immune response, prior to their occurrence in plasma. To investigate similar phenomena in a model of immunization against hepatitis B virus (HBV) infection, medical students in Ghana were screened for HBV markers, HBV surface (HBs) antigen (HBsAg), and HBV core antibodies (anti-HBc). Consenting volunteers, 24 of whom were seronegative (susceptible) and 2 of whom were positive for anti-HBc (prior infection), were vaccinated on day 0, day 40, and 6 months. Two sets of 10 blood samples, sequentially collected at intervals of 2 days following each immunization on days 0 and 40, were processed into B-cell lysates and plasma. Solid-phase HBsAg coated on microtiter plates for enzyme immunoassay or nitrocellulose membranes for dot blot assay was used to detect anti-HBs activity by an indirect antiglobulin assay. A commercially procured sandwich immunoassay was used, along with an enzyme-linked immunosorbent assay and a dot blot assay, for the detection of anti-HBs in B-cell lysates and plasma. Following the first injection of vaccine, a single sample of B-cell lysate collected between 5 and 21 days revealed anti-HBs in 18/21 subjects with no plasma antibodies detectable by sandwich immunoassay. After the booster dose was injected on day 40, a single sample of B-cell lysate collected between 44 and 49 days showed anti-HBs in 16/19 subjects, and this was accompanied by plasma antibodies in 8 subjects. In contrast, between 8 and 13 days, both subjects with prior HBV infection showed anti-HBs in B-cell lysates and plasma. Thus, primary immunization with the HBV vaccine appears to transiently elicit low-affinity anti-HBs in B-cell lysates into plasma.
Figures

Similar articles
-
Hepatitis B virus markers in anti-HBc only positive individuals.J Med Virol. 2001 Jul;64(3):312-9. doi: 10.1002/jmv.1052. J Med Virol. 2001. PMID: 11424120
-
Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study.BMC Public Health. 2014 Sep 23;14:991. doi: 10.1186/1471-2458-14-991. BMC Public Health. 2014. PMID: 25248369 Free PMC article.
-
Persistence of hepatitis B surface antibody and immune memory to hepatitis B vaccine among medical college students in Madinah.Ann Saudi Med. 2018 Nov-Dec;38(6):413-419. doi: 10.5144/0256-4947.2018.413. Ann Saudi Med. 2018. PMID: 30531175 Free PMC article.
-
Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen.J Biomed Sci. 2001 May-Jun;8(3):237-47. doi: 10.1007/BF02256597. J Biomed Sci. 2001. PMID: 11385295 Review.
-
Expression and detection of anti-HBs antibodies after hepatitis B virus infection or vaccination in the context of protective immunity.Arch Virol. 2019 Nov;164(11):2645-2658. doi: 10.1007/s00705-019-04369-9. Epub 2019 Aug 9. Arch Virol. 2019. PMID: 31399876 Review.
Cited by
-
Acute hepatitis B virus (HBV) infection in a repeat blood donor during anti-HBV vaccination.Blood Transfus. 2012 Jul;10(3):384-6. doi: 10.2450/2012.0094-11. Epub 2012 Mar 28. Blood Transfus. 2012. PMID: 22507859 Free PMC article. No abstract available.
-
Significance of anti-HBc only in blood donors: a serological and virological study after hepatitis B vaccination.Blood Transfus. 2014 Jan;12 Suppl 1(Suppl 1):s63-8. doi: 10.2450/2013.0227-12. Epub 2013 Feb 21. Blood Transfus. 2014. PMID: 23522882 Free PMC article.
-
Multiplexed Remote SPR Detection of Biological Interactions through Optical Fiber Bundles.Sensors (Basel). 2020 Jan 16;20(2):511. doi: 10.3390/s20020511. Sensors (Basel). 2020. PMID: 31963277 Free PMC article.
-
Early detection of human immunodeficiency virus type 1-specific B-lymphocyte-derived antibodies in a high-risk population.Clin Vaccine Immunol. 2009 Jul;16(7):1060-5. doi: 10.1128/CVI.00280-08. Epub 2009 May 27. Clin Vaccine Immunol. 2009. PMID: 19474262 Free PMC article.
References
-
- Ambrosino, D. M., M. V. Kanchana, N. R. Delaney, R. W. Finberg, and B. Hum. 1991. Cells secrete predominantly L chains in the absence of H chain expression. J. Immunol. 146:599-602. - PubMed
-
- Bocher, W. O., S. Herzog-Hauff, J. Schlaak, K. H. Meyer zum Buschenfelde, and H. F. Lohr. 1999. Kinetics of hepatitis B surface antigen-specific responses in acute and chronic hepatitis B or after HBs vaccination: stimulation of the in vitro antibody response by interferon gamma. Hepatology 29:238-244. - PubMed
-
- Cherlet, M., S. J. Kromenaker, and F. Srienct. 1995. Surface IgG content of murine hybridomas: direct evidence for variation of antibody secretion rates during the cell cycle. Biotech. Bioeng. 47:535-540. - PubMed
-
- Cox, R. J., K. A. Brokstad, and L. R. Haaheim. 1996. Kinetics of the early immune response induced after parenteral influenza vaccination, p. 561-571. In L. E. Brown, A. W. Hampson, and R. G. Webster (ed.), Options for the control of influenza III. Excerpta Medica, Elsevier, Amsterdam, The Netherlands.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

