Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex
- PMID: 17928435
- PMCID: PMC6672847
- DOI: 10.1523/JNEUROSCI.1629-07.2007
Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex
Abstract
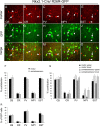
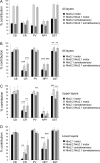
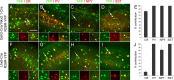
Cortical pyramidal cells are generated from pallial neuroepithelial precursors, whereas GABAergic interneurons originate in subpallial germinal zones and migrate tangentially to reach the cortex. Using Cre-lox technology in transgenic mice and a series of molecular markers that subdivide the subpallial neuroepithelium into small domains, we fate-map precursor pools and identify interneurons generated from each domain. Cortical interneurons expressing calbindin, parvalbumin, and somatostatin are generated exclusively from Lhx6 (Lim homeobox 6)-expressing precursors in the medial ganglionic eminence (MGE). Martinotti cells that coexpress calretinin and somatostatin are generated from the dorsal region of the MGE neuroepithelium that expresses Nkx6.2 (NK2 transcription factor-related 6.2). Most neuropeptide Y-expressing cells and all bipolar calretinin-expressing interneurons are generated outside the MGE, from the germinal zones of the lateral/caudal ganglionic eminences that express Gsh2 (genomic screened homeobox 2). Our data demonstrate that subpallial neuroepithelial domains defined by expression of genetic determinants generate distinct interneuron subtypes, thereby contributing to the generation of cortical interneuron heterogeneity observed in the adult cortex.
Figures





References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Bellion A, Wassef M, Metin C. Early differences in axonal outgrowth cell migration GABAergic differentiation properties between the dorsal lateral cortex. Cereb Cortex. 2003;13:203–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
