A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning
- PMID: 17928436
- PMCID: PMC6672841
- DOI: 10.1523/JNEUROSCI.2603-07.2007
A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning
Abstract
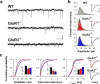
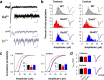
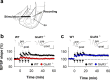
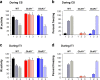
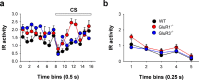
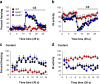
The AMPA receptor subunit glutamate receptor 1 (GluR1 or GluR-A) contributes to amygdala-dependent emotional learning. It remains unclear, however, to what extent different amygdala pathways depend on GluR1, or other AMPA receptor subunits, for proper synaptic transmission and plasticity, and whether GluR1-dependent long-term potentiation (LTP) is necessary for auditory and contextual fear conditioning. Here, we dissected the role of GluR1 and GluR3 (GluR-C) subunits in AMPA receptor-dependent amygdala LTP and fear conditioning using knock-out mice (GluR1-/- and GluR3-/-). We found that, whereas LTP at thalamic inputs to lateral amygdala (LA) projection neurons and at glutamatergic synapses in the basal amygdala was completely absent in GluR1-/- mice, both GluR1 and GluR3 contributed to LTP in the cortico-LA pathway. Because both auditory and contextual fear conditioning were selectively impaired in GluR1-/- but not GluR3-/- mice, we conclude that GluR1-dependent synaptic plasticity is the dominant form of LTP underlying the acquisition of auditory and contextual fear conditioning, and that plasticity in distinct amygdala pathways differentially contributes to aversive conditioning.
Figures



Similar articles
-
Auditory fear conditioning facilitates neurotransmitter release at lateral amygdala to basal amygdala synapses.Biochem Biophys Res Commun. 2021 Dec 20;584:39-45. doi: 10.1016/j.bbrc.2021.11.014. Epub 2021 Nov 8. Biochem Biophys Res Commun. 2021. PMID: 34768080
-
Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents.J Physiol. 2012 Jan 1;590(1):193-208. doi: 10.1113/jphysiol.2011.220434. Epub 2011 Nov 14. J Physiol. 2012. PMID: 22083603 Free PMC article.
-
Signaling through cGMP-dependent protein kinase I in the amygdala is critical for auditory-cued fear memory and long-term potentiation.J Neurosci. 2008 Dec 24;28(52):14202-12. doi: 10.1523/JNEUROSCI.2216-08.2008. J Neurosci. 2008. PMID: 19109502 Free PMC article.
-
Synaptic mechanisms of associative memory in the amygdala.Neuron. 2005 Sep 15;47(6):783-6. doi: 10.1016/j.neuron.2005.08.009. Neuron. 2005. PMID: 16157273 Review.
-
Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits.Mol Neurobiol. 1996 Aug;13(1):1-22. doi: 10.1007/BF02740749. Mol Neurobiol. 1996. PMID: 8892333 Review.
Cited by
-
Amygdala GluN2B-NMDAR dysfunction is critical in abnormal aggression of neurodevelopmental origin induced by St8sia2 deficiency.Mol Psychiatry. 2020 Sep;25(9):2144-2161. doi: 10.1038/s41380-018-0132-3. Epub 2018 Aug 8. Mol Psychiatry. 2020. PMID: 30089788 Free PMC article.
-
Synaptic maturation at cortical projections to the lateral amygdala in a mouse model of Rett syndrome.PLoS One. 2010 Jul 2;5(7):e11399. doi: 10.1371/journal.pone.0011399. PLoS One. 2010. PMID: 20625482 Free PMC article.
-
The selectivity of aversive memory reconsolidation and extinction processes depends on the initial encoding of the Pavlovian association.Learn Mem. 2013 Nov 19;20(12):695-9. doi: 10.1101/lm.031609.113. Learn Mem. 2013. PMID: 24255099 Free PMC article.
-
Synaptic Mechanisms of Delay Eyeblink Classical Conditioning: AMPAR Trafficking and Gene Regulation in an In Vitro Model.Mol Neurobiol. 2023 Dec;60(12):7088-7103. doi: 10.1007/s12035-023-03528-z. Epub 2023 Aug 2. Mol Neurobiol. 2023. PMID: 37531025 Review.
-
Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment.Neuropsychopharmacology. 2019 Jun;44(7):1247-1257. doi: 10.1038/s41386-019-0340-9. Epub 2019 Feb 13. Neuropsychopharmacology. 2019. PMID: 30758321 Free PMC article.
References
-
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. - PubMed
-
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. - PubMed
-
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
