Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia
- PMID: 17928440
- PMCID: PMC4024474
- DOI: 10.1523/JNEUROSCI.2357-07.2007
Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia
Abstract
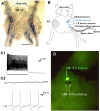
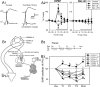
Nitric oxide (NO) is thought to be involved in several forms of learning in vivo and synaptic plasticity in vitro, but very little is known about the role of NO during physiological forms of plasticity that occur during learning. We addressed that question in a simplified preparation of the Aplysia siphon-withdrawal reflex. We first used in situ hybridization to show that the identified L29 facilitator neurons express NO synthase. Furthermore, exogenous NO produced facilitation of sensory-motor neuron EPSPs, and an inhibitor of NO synthase or an NO scavenger blocked behavioral conditioning. Application of the scavenger to the ganglion or injection into a sensory neuron blocked facilitation of the EPSP and changes in the sensory-neuron membrane properties during conditioning. Injection of the scavenger into the motor neuron reduced facilitation without affecting sensory neuron membrane properties, and injection of an inhibitor of NO synthase had no effect. Postsynaptic injection of an inhibitor of exocytosis had effects similar to injection of the scavenger. However, changes in the shape of the EPSP during conditioning were not consistent with postsynaptic AMPA-like receptor insertion but were mimicked by presynaptic spike broadening. These results suggest that NO makes an important contribution during conditioning and acts directly in both the sensory and motor neurons to affect different processes of facilitation at the synapses between them. In addition, they suggest that NO does not come from either the sensory or motor neurons but rather comes from another source, perhaps the L29 interneurons.
Figures







References
-
- Abrams TW, Yovell Y, Onyike CU, Cohen JE, Jarrard HE. Analysis of sequence-dependent interactions between calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: possible contributions to CS-US sequence requirement during conditioning. Learn Mem. 1998;4:496–509. - PubMed
-
- Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. - PubMed
-
- Antonova I, Arancio O, Trillat A-C, Wang H-G, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
