Huntingtin-interacting protein 1 influences worm and mouse presynaptic function and protects Caenorhabditis elegans neurons against mutant polyglutamine toxicity
- PMID: 17928447
- PMCID: PMC6672856
- DOI: 10.1523/JNEUROSCI.1941-07.2007
Huntingtin-interacting protein 1 influences worm and mouse presynaptic function and protects Caenorhabditis elegans neurons against mutant polyglutamine toxicity
Abstract
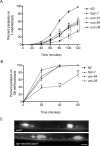
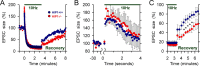
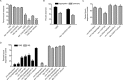
Huntingtin-interacting protein 1 (HIP1) was identified through its interaction with htt (huntingtin), the Huntington's disease (HD) protein. HIP1 is an endocytic protein that influences transport and function of AMPA and NMDA receptors in the brain. However, little is known about its contribution to neuronal dysfunction in HD. We report that the Caenorhabditis elegans HIP1 homolog hipr-1 modulates presynaptic activity and the abundance of synaptobrevin, a protein involved in synaptic vesicle fusion. Presynaptic function was also altered in hippocampal brain slices of HIP1-/- mice demonstrating delayed recovery from synaptic depression and a reduction in paired-pulse facilitation, a form of presynaptic plasticity. Interestingly, neuronal dysfunction in transgenic nematodes expressing mutant N-terminal huntingtin was specifically enhanced by hipr-1 loss of function. A similar effect was observed with several other mutant proteins that are expressed at the synapse and involved in endocytosis, such as unc-11/AP180, unc-26/synaptojanin, and unc-57/endophilin. Thus, HIP1 is involved in presynaptic nerve terminal activity and modulation of mutant polyglutamine-induced neuronal dysfunction. Moreover, synaptic proteins involved in endocytosis may protect neurons against amino acid homopolymer expansion.
Figures



References
-
- Abenavoli A, Montagna M, Malgaroli A. Calcium: the common theme in vesicular cycling. Nat Neurosci. 2001;4:117–118. - PubMed
-
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. - PubMed
-
- Crocker SF, Costain WJ, Robertson HA. DNA microarray analysis of striatal gene expression in symptomatic transgenic Huntington's mice (R6/2) reveals neuroinflammation and insulin associations. Brain Res. 2006;1088:176–186. - PubMed
-
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
