Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects
- PMID: 17928532
- PMCID: PMC2200850
- DOI: 10.1182/blood-2007-05-089573
Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects
Abstract
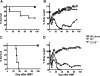
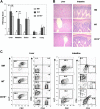
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in allogeneic hematopoietic stem cell transplantation. Migration of donor-derived T cells into GVHD target organs plays an essential role in the development of GVHD. beta2 integrins are critically important for leukocyte extravasation through vascular endothelia and for T-cell activation. We asked whether CD18-deficient T cells would induce less GVHD while sparing the graft-versus-leukemia (GVL) effect. In murine allogeneic bone marrow transplantation models, we found that recipients of CD18-/- donor T cells had significantly less GVHD morbidity and mortality compared with recipients of wild-type (WT) donor T cells. Analysis of alloreactivity showed that CD18-/- and WT T cells had comparable activation, expansion, and cytokine production in vivo. Reduced GVHD was associated with a significant decrease in donor T-cell infiltration of recipient intestine and with an overall decrease in pathologic scores in intestine and liver. Finally, we found that the in vivo GVL effect of CD18-/- donor T cells was largely preserved, because mortality of the recipients who received transplants of CD18-/- T cells plus tumor cells was greatly delayed or prevented. Our data suggest that strategies to target beta2 integrin have clinical potential to alleviate or prevent GVHD while sparing GVL activity.
Figures


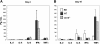

Similar articles
-
Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect.Blood. 2001 May 1;97(9):2886-95. doi: 10.1182/blood.v97.9.2886. Blood. 2001. PMID: 11313285
-
Differential Effect of MyD88 Signal in Donor T Cells on Graft-versus-Leukemia Effect and Graft-versus-Host Disease after Experimental Allogeneic Stem Cell Transplantation.Mol Cells. 2015 Nov;38(11):966-74. doi: 10.14348/molcells.2015.0158. Epub 2015 Nov 10. Mol Cells. 2015. PMID: 26552489 Free PMC article.
-
Ex vivo fludarabine exposure inhibits graft-versus-host activity of allogeneic T cells while preserving graft-versus-leukemia effects.Biol Blood Marrow Transplant. 2003 Oct;9(10):616-32. doi: 10.1016/s1083-8791(03)00229-5. Biol Blood Marrow Transplant. 2003. PMID: 14569558
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
-
Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease.Front Immunol. 2018 Dec 21;9:3041. doi: 10.3389/fimmu.2018.03041. eCollection 2018. Front Immunol. 2018. PMID: 30619371 Free PMC article. Review.
Cited by
-
CD8(+) Tregs promote GVHD prevention and overcome the impaired GVL effect mediated by CD4(+) Tregs in mice.Oncoimmunology. 2016 Mar 28;5(6):e1146842. doi: 10.1080/2162402X.2016.1146842. eCollection 2016 Jun. Oncoimmunology. 2016. PMID: 27471614 Free PMC article.
-
Bim is required for T-cell allogeneic responses and graft-versus-host disease in vivo.Am J Blood Res. 2012;2(1):77-85. Epub 2012 Jan 1. Am J Blood Res. 2012. PMID: 22432091 Free PMC article.
-
PIK3CB is involved in metastasis through the regulation of cell adhesion to collagen I in pancreatic cancer.J Adv Res. 2021 Feb 17;33:127-140. doi: 10.1016/j.jare.2021.02.002. eCollection 2021 Nov. J Adv Res. 2021. PMID: 34603784 Free PMC article.
-
Low-density array PCR analysis of reperfusion biopsies: an adjunct to histological analysis.Nephrol Dial Transplant. 2010 Dec;25(12):4077-86. doi: 10.1093/ndt/gfq297. Epub 2010 May 26. Nephrol Dial Transplant. 2010. PMID: 20504838 Free PMC article.
-
LBH589 enhances T cell activation in vivo and accelerates graft-versus-host disease in mice.Biol Blood Marrow Transplant. 2012 Aug;18(8):1182-1190.e1. doi: 10.1016/j.bbmt.2012.06.002. Epub 2012 Jun 12. Biol Blood Marrow Transplant. 2012. PMID: 22698484 Free PMC article.
References
-
- Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. - PubMed
-
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. - PubMed
-
- Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–117. - PubMed
-
- Bachmann MF, McKall-Faienza K, Schmits R, et al. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. - PubMed
-
- Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

