Preclinical and clinical in vitro in vivo correlation of an hGH dextran microsphere formulation
- PMID: 17929148
- PMCID: PMC2063566
- DOI: 10.1007/s11095-007-9433-y
Preclinical and clinical in vitro in vivo correlation of an hGH dextran microsphere formulation
Abstract
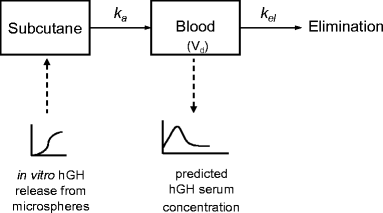
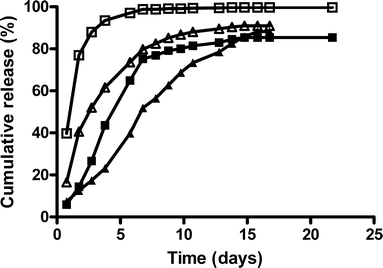
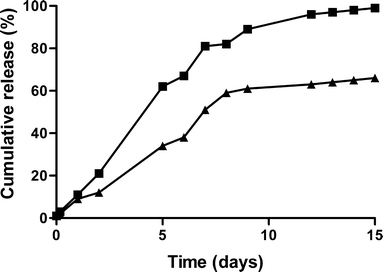
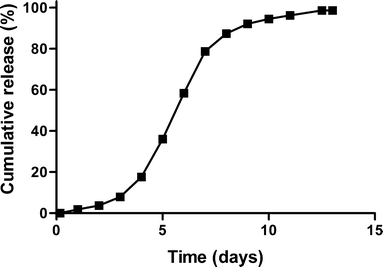
Purpose: To investigate the in vitro in vivo correlation of a sustained release formulation for human growth hormone (hGH) based on hydroxyethyl methacrylated dextran (dex-HEMA) microspheres in Pit-1 deficient Snell dwarf mice and in healthy human volunteers.
Materials and methods: A hGH-loaded microsphere formulation was developed and tested in Snell dwarf mice (pharmacodynamic study) and in healthy human volunteers (pharmacokinetic study).
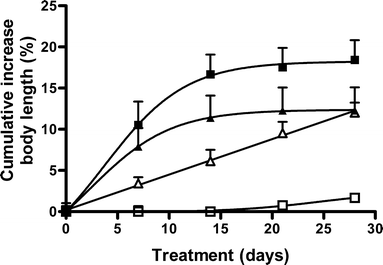
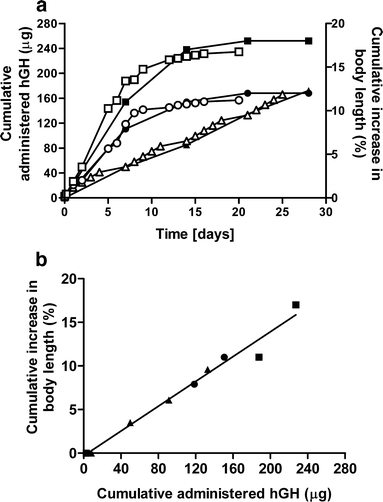
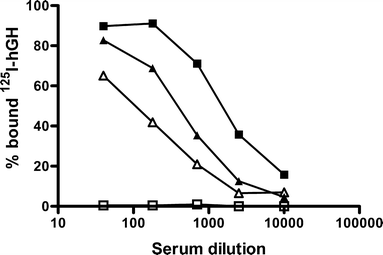
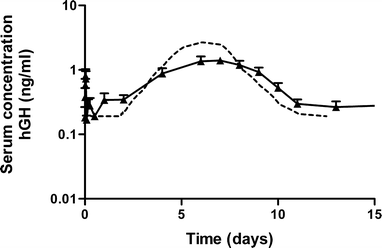
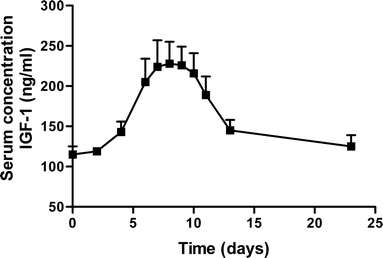
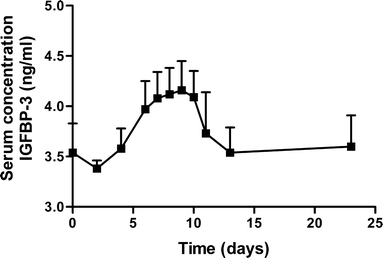
Results: Single subcutaneous administration of the microspheres in mice resulted in a good correlation between hGH released in vitro and in vivo effects for the hGH-loaded microsphere formulation similar to daily injected hGH indicating a retained bioactivity. Testing the microspheres in healthy volunteers showed an increase (over 7-8 days) in hGH serum concentrations (peak concentrations: 1-2.5 ng/ml). A good in vitro in vivo correlation was obtained between the measured and calculated (from in vitro release data) hGH serum concentrations. Moreover, an increased serum concentration of biomarkers (insulin-like growth factor-I (IGF-I), IGF binding protein-3 (IGFBP-3) was found again indicating that bioactive hGH was released from the microspheres.
Conclusions: Good in vitro in vivo correlations were obtained for hGH-loaded dex-HEMA microspheres, which is an important advantage in predicting the effect of the controlled drug delivery product in a clinical situations.
Figures
Similar articles
-
Sustained release hGH microsphere formulation produced by a novel supercritical fluid technology: in vivo studies.J Control Release. 2010 Jan 25;141(2):153-60. doi: 10.1016/j.jconrel.2009.09.013. Epub 2009 Sep 20. J Control Release. 2010. PMID: 19772878
-
Development of Recombinant Human Growth Hormone (rhGH) sustained-release microspheres by a low temperature aqueous phase/aqueous phase emulsion method.Eur J Pharm Sci. 2014 Oct 1;62:141-7. doi: 10.1016/j.ejps.2014.05.027. Epub 2014 Jun 4. Eur J Pharm Sci. 2014. PMID: 24907681
-
Characterization and in vivo study of sustained-release formulation of human growth hormone using sodium hyaluronate.Pharm Res. 2004 Aug;21(8):1374-81. doi: 10.1023/b:pham.0000036910.41224.de. Pharm Res. 2004. PMID: 15359571
-
Sustained delivery of human growth hormone using a polyelectrolyte complex-loaded thermosensitive polyphosphazene hydrogel.J Control Release. 2010 Nov 1;147(3):359-67. doi: 10.1016/j.jconrel.2010.07.126. Epub 2010 Aug 14. J Control Release. 2010. PMID: 20713099
-
Points to consider when establishing drug product specifications for parenteral microspheres.AAPS J. 2010 Mar;12(1):27-32. doi: 10.1208/s12248-009-9156-6. Epub 2009 Nov 17. AAPS J. 2010. PMID: 19921439 Free PMC article. Review.
Cited by
-
IVIVC of Octreotide in PLGA-Glucose Microsphere Formulation, Sandostatin® LAR.AAPS PharmSciTech. 2022 Sep 19;23(7):258. doi: 10.1208/s12249-022-02359-w. AAPS PharmSciTech. 2022. PMID: 36123513
-
Preparation and in vivo/in vitro characterization of Ticagrelor PLGA sustained-release microspheres for injection.Des Monomers Polym. 2021 Oct 8;24(1):305-319. doi: 10.1080/15685551.2021.1984008. eCollection 2021. Des Monomers Polym. 2021. PMID: 34650328 Free PMC article.
-
In vitro-in vivo correlation for complex non-oral drug products: Where do we stand?J Control Release. 2015 Dec 10;219:644-651. doi: 10.1016/j.jconrel.2015.09.052. Epub 2015 Sep 28. J Control Release. 2015. PMID: 26419305 Free PMC article. Review.
-
Patient-Centric Long-Acting Injectable and Implantable Platforms─An Industrial Perspective.Mol Pharm. 2024 Sep 2;21(9):4238-4258. doi: 10.1021/acs.molpharmaceut.4c00665. Epub 2024 Aug 19. Mol Pharm. 2024. PMID: 39160132 Free PMC article. Review.
-
Growth hormone receptor modulators.Rev Endocr Metab Disord. 2009 Jun;10(2):145-56. doi: 10.1007/s11154-008-9089-x. Rev Endocr Metab Disord. 2009. PMID: 18622706 Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0168-3659(97)00075-8', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0168-3659(97)00075-8'}]}
- J. L. Cleland, E. Duenas, A. Daugherty, A. Marian, J. Yang, M. Wilson, A. C. Celniker, A. Shahzamani, V. Quarmby, H. Chu, V. Mukku, A. Mac, M. Roussakis, N. Gillette, B. Brooks, D. Yueng, D. Brooks, Y.-F. Maa, C. Hsu, and J. S. Jones. Recombinant human growth hormone poly(lactic-co-glycolic acid) (PLGA) microspheres provide long lasting effect. J. Control. Rel.49:193–205 (1997).
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/nm0796-795', 'is_inner': False, 'url': 'https://doi.org/10.1038/nm0796-795'}, {'type': 'PubMed', 'value': '8673926', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8673926/'}]}
- O. L. Johnson, J. L. Cleland, H. J. Lee, M. Charnis, E. Duenas, W. Jaworowicz, D. Shepard, A. Shahzamani, A. J. S. Jones, and S. D. Putney. A month-long effect from a single injection of microencapsulated human growth hormone. Nat. Med.2:795–799 (1996). - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0168-3659(97)01648-9', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0168-3659(97)01648-9'}]}
- M. Katakam, W. R. Ravis, and A. K. Banga. Controlled release of human growth hormone in rats following parenteral administration of poloxamer gels. J. Control. Rel.49:21–26 (1997).
-
- None
- M. Reslow, M. Joensson, and T. Laakso. Sustained-release of human growth hormone from PLG-coated starch microspheres. Drug Del. Sys. Sci.2:103–109 (2002).
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0168-3659(02)00494-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0168-3659(02)00494-7'}, {'type': 'PubMed', 'value': '12628330', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12628330/'}]}
- S. Takada, Y. Yamagata, M. Misaki, K. Taira, and T. Kurokawa. Sustained release of human growth hormone from microcapsules prepared by a solvent evaporation technique. J. Control. Release88:229–242 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

