Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs
- PMID: 17931426
- PMCID: PMC2225395
- DOI: 10.1186/1746-4811-3-12
Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs
Abstract
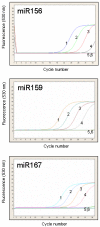
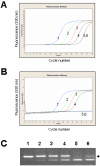
MicroRNAs (miRNAs) are a class of small non-coding RNAs with a critical role in development and environmental responses. Efficient and reliable detection of miRNAs is an essential step towards understanding their roles in specific cells and tissues. However, gel-based assays currently used to detect miRNAs are very limited in terms of throughput, sensitivity and specificity. Here we provide protocols for detection and quantification of miRNAs by RT-PCR. We describe an end-point and real-time looped RT-PCR procedure and demonstrate detection of miRNAs from as little as 20 pg of plant tissue total RNA and from total RNA isolated from as little as 0.1 mul of phloem sap. In addition, we have developed an alternative real-time PCR assay that can further improve specificity when detecting low abundant miRNAs. Using this assay, we have demonstrated that miRNAs are differentially expressed in the phloem sap and the surrounding vascular tissue. This method enables fast, sensitive and specific miRNA expression profiling and is suitable for facilitation of high-throughput detection and quantification of miRNA expression.
Figures


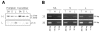
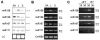

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

