Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A
- PMID: 17932105
- PMCID: PMC2234582
- DOI: 10.1210/me.2007-0369
Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A
Abstract
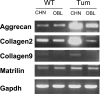
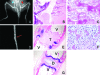
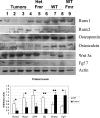
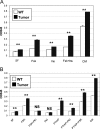
Carney complex (CNC) is an autosomal dominant neoplasia syndrome caused by inactivating mutations in PRKAR1A, the gene encoding the type 1A regulatory subunit of protein kinase A (PKA). This genetic defect induces skin pigmentation, endocrine tumors, myxomas, and schwannomas. Some patients with the complex also develop myxoid bone tumors termed osteochondromyxomas. To study the link between the PRKAR1A mutations and tumor formation, we generated a mouse model of this condition. Prkar1a(+/-) mice develop bone tumors with high frequency, although these lesions have not yet been characterized, either from human patients or from mice. Bone tumors from Prkar1a(+/-) mice were heterogeneous, including elements of myxomatous, cartilaginous, and bony differentiation that effaced the normal bone architecture. Immunohistochemical analysis identified an osteoblastic origin for the abnormal cells associated with islands of bone. To better understand these cells at the biochemical level, we isolated primary cultures of tumoral bone and compared them with cultures of bone from wild-type animals. The tumor cells exhibited the expected decrease in Prkar1a protein and exhibited increased PKA activity. At the phenotypic level, we observed that tumor cells behaved as incompletely differentiated osteoblasts and were able to form tumors in immunocompromised mice. Examination of gene expression revealed down-regulation of markers of bone differentiation and increased expression of locally acting growth factors, including members of the Wnt signaling pathway. Tumor cells exhibited enhanced growth in response to PKA-stimulating agents, suggesting that tumorigenesis in osteoblast precursor cells is driven by effects directly mediated by the dysregulation of PKA.
Figures



Similar articles
-
Haploinsufficiency for either one of the type-II regulatory subunits of protein kinase A improves the bone phenotype of Prkar1a+/- mice.Hum Mol Genet. 2015 Nov 1;24(21):6080-92. doi: 10.1093/hmg/ddv320. Epub 2015 Aug 5. Hum Mol Genet. 2015. PMID: 26246497 Free PMC article.
-
Use of mouse models to understand the molecular basis of tissue-specific tumorigenesis in the Carney complex.J Intern Med. 2009 Jul;266(1):60-8. doi: 10.1111/j.1365-2796.2009.02114.x. J Intern Med. 2009. PMID: 19522826 Review.
-
Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit (PRKAR1A) in patients with the "complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas" (Carney complex).Ann N Y Acad Sci. 2002 Jun;968:3-21. doi: 10.1111/j.1749-6632.2002.tb04323.x. Ann N Y Acad Sci. 2002. PMID: 12119264 Review.
-
A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues.Cancer Res. 2005 Jun 1;65(11):4506-14. doi: 10.1158/0008-5472.CAN-05-0580. Cancer Res. 2005. PMID: 15930266
-
Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells.Cancer Res. 2009 Sep 15;69(18):7278-84. doi: 10.1158/0008-5472.CAN-09-1601. Epub 2009 Sep 8. Cancer Res. 2009. PMID: 19738044
Cited by
-
Haploinsufficiency for either one of the type-II regulatory subunits of protein kinase A improves the bone phenotype of Prkar1a+/- mice.Hum Mol Genet. 2015 Nov 1;24(21):6080-92. doi: 10.1093/hmg/ddv320. Epub 2015 Aug 5. Hum Mol Genet. 2015. PMID: 26246497 Free PMC article.
-
Suppressing the activation of protein kinase A as a DNA damage-independent mechanistic lead for dihydromethysticin prophylaxis of NNK-induced lung carcinogenesis.Carcinogenesis. 2022 Aug 30;43(7):659-670. doi: 10.1093/carcin/bgac031. Carcinogenesis. 2022. PMID: 35353881 Free PMC article.
-
Osteochondromyxoma: Review of a rare carney complex criterion.J Bone Oncol. 2016 Jul 12;5(4):194-197. doi: 10.1016/j.jbo.2016.07.002. eCollection 2016 Nov. J Bone Oncol. 2016. PMID: 28008382 Free PMC article. Review.
-
Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone.Proc Natl Acad Sci U S A. 2010 May 11;107(19):8683-8. doi: 10.1073/pnas.1003680107. Epub 2010 Apr 26. Proc Natl Acad Sci U S A. 2010. PMID: 20421483 Free PMC article.
-
Knockdown of PRKAR1A, the gene responsible for Carney complex, interferes with differentiation in osteoblastic cells.Mol Endocrinol. 2014 Mar;28(3):295-307. doi: 10.1210/me.2013-1152. Epub 2014 Feb 7. Mol Endocrinol. 2014. PMID: 24506536 Free PMC article.
References
-
- Carney JA 1995 The Carney complex (myxomas, spotty pigmentation, endocrine overactivity, and schwannomas). Dermatol Clin 13:19–26 - PubMed
-
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL 1985 The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64:270–283 - PubMed
-
- Kirschner LS, Stratakis CA 2002 Genetic analysis of Carney complex: current understanding and future questions. Curr Opin Endocrinol Diabetes 9:244–249
-
- Carney JA, Boccon-Gibod L, Jarka DE, Tanaka Y, Swee RG, Unni KK, Stratakis CA 2001 Osteochondromyxoma of bone: a congenital tumor associated with lentigines and other unusual disorders. Am J Surg Pathol 25:164–176 - PubMed
-
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000 Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

