Voltage is a partial activator of rat thermosensitive TRP channels
- PMID: 17932142
- PMCID: PMC2375500
- DOI: 10.1113/jphysiol.2007.144287
Voltage is a partial activator of rat thermosensitive TRP channels
Abstract
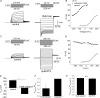
TRPV1 and TRPM8 are sensory nerve ion channels activated by heating and cooling, respectively. A variety of physical and chemical stimuli activate these receptors in a synergistic manner but the underlying mechanisms are unclear. Both channels are voltage sensitive, and temperature and ligands modulate this voltage dependence. Thus, a voltage-sensing mechanism has become an attractive model to explain the generalized gating of these and other thermo-sensitive TRP channels. We show here using whole-cell and single channel measurements that voltage produces only a partial activation of TRPV1 and TRPM8. At room temperature (20-25 degrees C) membrane depolarization evokes responses that saturate at approximately 50-60% of the maximum open probability. Furthermore, high concentrations of capsaicin (10 microm), resiniferatoxin (5 microm) and menthol (6 mm) reveal voltage-independent gating. Similarly, other modes of TRPV1 regulation including heat, protein kinase C-dependent phosphorylation, and protons enhance both the efficacy and sensitivity of voltage activation. In contrast, the TRPV1 antagonist capsazepine produces the opposite effects. These data can be explained by an allosteric model in which voltage, temperature, agonists and inverse agonists are independently coupled, either positively or negatively, to channel gating. Thus, voltage acts separately but in concert with other stimuli to regulate channel activation, and, therefore, a voltage-sensitive mechanism is unlikely to represent a final, gating mechanism for these channels.
Figures
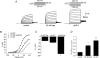




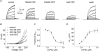


Comment in
-
Understanding the role of voltage gating of polymodal TRP channels.J Physiol. 2007 Dec 1;585(Pt 2):321-2. doi: 10.1113/jphysiol.2007.147082. J Physiol. 2007. PMID: 18056113 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

