Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae
- PMID: 17932304
- PMCID: PMC2151694
- DOI: 10.1104/pp.107.108480
Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae
Abstract
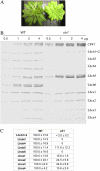
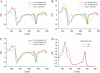
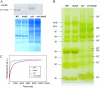
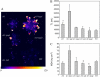
The ch1 mutant of Arabidopsis (Arabidopsis thaliana) lacks chlorophyll (Chl) b. Leaves of this mutant are devoid of photosystem II (PSII) Chl-protein antenna complexes and have a very low capacity of nonphotochemical quenching (NPQ) of Chl fluorescence. Lhcb5 was the only PSII antenna protein that accumulated to a significant level in ch1 mutant leaves, but the apoprotein did not assemble in vivo with Chls to form a functional antenna. The abundance of Lhca proteins was also reduced to approximately 20% of the wild-type level. ch1 was crossed with various xanthophyll mutants to analyze the antioxidant activity of carotenoids unbound to PSII antenna. Suppression of zeaxanthin by crossing ch1 with npq1 resulted in oxidative stress in high light, while removing other xanthophylls or the PSII protein PsbS had no such effect. The tocopherol-deficient ch1 vte1 double mutant was as sensitive to high light as ch1 npq1, and the triple mutant ch1 npq1 vte1 exhibited an extreme sensitivity to photooxidative stress, indicating that zeaxanthin and tocopherols have cumulative effects. Conversely, constitutive accumulation of zeaxanthin in the ch1 npq2 double mutant led to an increased phototolerance relative to ch1. Comparison of ch1 npq2 with another zeaxanthin-accumulating mutant (ch1 lut2) that lacks lutein suggests that protection of polyunsaturated lipids by zeaxanthin is enhanced when lutein is also present. During photooxidative stress, alpha-tocopherol noticeably decreased in ch1 npq1 and increased in ch1 npq2 relative to ch1, suggesting protection of vitamin E by high zeaxanthin levels. Our results indicate that the antioxidant activity of zeaxanthin, distinct from NPQ, can occur in the absence of PSII light-harvesting complexes. The capacity of zeaxanthin to protect thylakoid membrane lipids is comparable to that of vitamin E but noticeably higher than that of all other xanthophylls of Arabidopsis leaves.
Figures



Similar articles
-
A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26.Plant Cell. 2005 Apr;17(4):1217-32. doi: 10.1105/tpc.104.030601. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749754 Free PMC article.
-
Photosynthesis, chlorophyll fluorescence, light-harvesting system and photoinhibition resistance of a zeaxanthin-accumulating mutant of Arabidopsis thaliana.J Photochem Photobiol B. 1996 Jun;34(1):87-94. doi: 10.1016/1011-1344(95)07272-1. J Photochem Photobiol B. 1996. PMID: 8765663
-
The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana.J Biol Chem. 2004 Apr 2;279(14):13878-88. doi: 10.1074/jbc.M311154200. Epub 2004 Jan 13. J Biol Chem. 2004. PMID: 14722117
-
The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II.Biochim Biophys Acta. 2012 Jan;1817(1):182-93. doi: 10.1016/j.bbabio.2011.04.012. Epub 2011 May 1. Biochim Biophys Acta. 2012. PMID: 21565154 Review.
-
Genetic manipulation of carotenoid biosynthesis and photoprotection.Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1395-403. doi: 10.1098/rstb.2000.0701. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11127994 Free PMC article. Review.
Cited by
-
Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits.J Biol Chem. 2012 Dec 7;287(50):41820-34. doi: 10.1074/jbc.M112.405498. Epub 2012 Oct 12. J Biol Chem. 2012. PMID: 23066020 Free PMC article.
-
LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco.Plant Physiol. 2012 Dec;160(4):1923-39. doi: 10.1104/pp.112.206045. Epub 2012 Oct 19. Plant Physiol. 2012. PMID: 23085838 Free PMC article.
-
Photoprotective Role of Neoxanthin in Plants and Algae.Molecules. 2020 Oct 11;25(20):4617. doi: 10.3390/molecules25204617. Molecules. 2020. PMID: 33050573 Free PMC article. Review.
-
Models and measurements of energy-dependent quenching.Photosynth Res. 2013 Oct;116(2-3):389-409. doi: 10.1007/s11120-013-9857-7. Epub 2013 Jun 23. Photosynth Res. 2013. PMID: 23793348 Free PMC article. Review.
-
Viral Perturbation of Alternative Splicing of a Host Transcript Benefits Infection.Plant Physiol. 2020 Nov;184(3):1514-1531. doi: 10.1104/pp.20.00903. Epub 2020 Sep 21. Plant Physiol. 2020. PMID: 32958561 Free PMC article.
References
-
- Bassi R (1985) Spectral properties and polypeptide composition of the chlorophyll-proteins from granal and agranal chloroplasts of maize (Zea mays L.). Carlsberg Res Commun 50 127–143
-
- Bassi R, Hinz U, Barbato R (1985) The role of light harvesting complex and photosystem II in thylakoid stacking in the chlorina-f2 barley mutant. Carlsberg Res Commun 50 347–367
-
- Bassi R, Marquardt J, Lavergne J (1995) Biochemical and functional properties of photosystem II in agranal membranes from maize mesophyll and bundle sheath chloroplasts. Eur J Biochem 233 709–719 - PubMed
-
- Böhm F, Edge R, Land EJ, McGarvey DJ, Truscott TG (1997) Carotenoids enhance vitamin E antioxidant efficiency. J Am Chem Soc 119 621–622
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

