The isoenzyme 7 of tobacco NAD(H)-dependent glutamate dehydrogenase exhibits high deaminating and low aminating activities in vivo
- PMID: 17932305
- PMCID: PMC2151676
- DOI: 10.1104/pp.107.107813
The isoenzyme 7 of tobacco NAD(H)-dependent glutamate dehydrogenase exhibits high deaminating and low aminating activities in vivo
Abstract
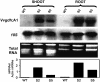
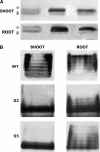
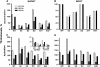
Following the discovery of glutamine synthetase/glutamate (Glu) synthase, the physiological roles of Glu dehydrogenase (GDH) in nitrogen metabolism in plants remain obscure and is the subject of considerable controversy. Recently, transgenics were used to overexpress the gene encoding for the beta-subunit polypeptide of GDH, resulting in the GDH-isoenzyme 1 deaminating in vivo Glu. In this work, we present transgenic tobacco (Nicotiana tabacum) plants overexpressing the plant gdh gene encoding for the alpha-subunit polypeptide of GDH. The levels of transcript correlated well with the levels of total GDH protein, the alpha-subunit polypeptide, and the abundance of GDH-anionic isoenzymes. Assays of transgenic plant extracts revealed high in vitro aminating and low deaminating activities. However, gas chromatography/mass spectrometry analysis of the metabolic fate of (15)NH(4) or [(15)N]Glu revealed that GDH-isoenzyme 7 mostly deaminates Glu and also exhibits low ammonium assimilating activity. These and previous results firmly establish the direction of the reactions catalyzed by the anionic and cationic isoenzymes of GDH in vivo under normal growth conditions and reveal a paradox between the in vitro and in vivo enzyme activities.
Figures
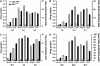

Similar articles
-
Resolving the role of plant glutamate dehydrogenase. I. In vivo real time nuclear magnetic resonance spectroscopy experiments.Plant Cell Physiol. 2009 Oct;50(10):1761-73. doi: 10.1093/pcp/pcp118. Epub 2009 Aug 18. Plant Cell Physiol. 2009. PMID: 19690000 Free PMC article.
-
Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine.Plant Cell. 2006 Oct;18(10):2767-81. doi: 10.1105/tpc.105.038323. Epub 2006 Oct 13. Plant Cell. 2006. PMID: 17041150 Free PMC article.
-
Tobacco isoenzyme 1 of NAD(H)-dependent glutamate dehydrogenase catabolizes glutamate in vivo.Plant Physiol. 2007 Jan;143(1):530-9. doi: 10.1104/pp.106.091330. Epub 2006 Nov 17. Plant Physiol. 2007. PMID: 17114271 Free PMC article.
-
Regulation of human glutamate dehydrogenases: implications for glutamate, ammonia and energy metabolism in brain.J Neurosci Res. 2001 Dec 1;66(5):899-908. doi: 10.1002/jnr.10054. J Neurosci Res. 2001. PMID: 11746417 Review.
-
Intertissue differences for the role of glutamate dehydrogenase in metabolism.Neurochem Res. 2014;39(3):516-26. doi: 10.1007/s11064-013-0998-z. Epub 2013 Feb 15. Neurochem Res. 2014. PMID: 23412807 Review.
Cited by
-
Nitrogen metabolic rate and differential ammonia volatilization regulate resistance against opportunistic fungus Alternaria alternata in tobacco.Front Plant Sci. 2022 Sep 23;13:1003534. doi: 10.3389/fpls.2022.1003534. eCollection 2022. Front Plant Sci. 2022. PMID: 36212279 Free PMC article.
-
The phosphorylated pathway of serine biosynthesis links plant growth with nitrogen metabolism.Plant Physiol. 2021 Jul 6;186(3):1487-1506. doi: 10.1093/plphys/kiab167. Plant Physiol. 2021. PMID: 34624108 Free PMC article.
-
Molecular analyses of the rice glutamate dehydrogenase gene family and their response to nitrogen and phosphorous deprivation.Plant Cell Rep. 2009 Jul;28(7):1115-26. doi: 10.1007/s00299-009-0709-z. Epub 2009 May 9. Plant Cell Rep. 2009. PMID: 19430792
-
Abiotic Stresses Downregulate Key Genes Involved in Nitrogen Uptake and Assimilation in Brassica juncea L.PLoS One. 2015 Nov 25;10(11):e0143645. doi: 10.1371/journal.pone.0143645. eCollection 2015. PLoS One. 2015. PMID: 26605918 Free PMC article.
-
Resolving the role of plant glutamate dehydrogenase. I. In vivo real time nuclear magnetic resonance spectroscopy experiments.Plant Cell Physiol. 2009 Oct;50(10):1761-73. doi: 10.1093/pcp/pcp118. Epub 2009 Aug 18. Plant Cell Physiol. 2009. PMID: 19690000 Free PMC article.
References
-
- Ameziane RK, Bernhard RB, Lightfoot D (2000) Expression of the bacterial gdhA gene encoding NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 221 47–57
-
- Aubert S, Bligny R, Douce R, Ratcliffe RG, Roberts JKM (2001) Contribution of glutamate dehydrogenase to mitochondrial metabolism studied by 13C and 31P nuclear magnetic resonance. J Exp Bot 52 37–45 - PubMed
-
- Bechtold U, Pahlich E, Lea PJ (1998) Methionine sulphoximine does not inhibit pea and wheat glutamate dehydrogenase. Phytochemistry 49 347–354
-
- Burum BP, Ernst RR (1980) Net polarization transfer via a J-ordered state for signal enhancement of low-sensitivity nuclei. J Magn Reson 39 163–168
-
- Cammaerts D, Jacobs M (1985) A study of the role of glutamate dehydrogenase (EC 1.4.1.2) in the nitrogen metabolism of Arabidopsis thaliana. Planta 163 517–526 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

