LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems
- PMID: 17932562
- PMCID: PMC2000807
- DOI: 10.1172/JCI31184
LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems
Abstract
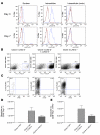
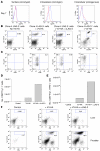
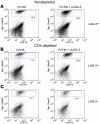
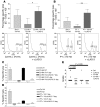
Lymphocyte activation gene-3 (LAG-3) is a cell-surface molecule with diverse biologic effects on T cell function. We recently showed that LAG-3 signaling is important in CD4+ regulatory T cell suppression of autoimmune responses. Here, we demonstrate that LAG-3 maintains tolerance to self and tumor antigens via direct effects on CD8+ T cells using 2 murine systems. Naive CD8+ T cells express low levels of LAG-3, and expression increases upon antigen stimulation. Our data show increased levels of LAG-3 protein on antigen-specific CD8+ T cells within antigen-expressing organs or tumors. In vivo antibody blockade of LAG-3 or genetic ablation of the Lag-3 gene resulted in increased accumulation and effector function of antigen-specific CD8+ T cells within organs and tumors that express their cognate antigen. Most notably, combining LAG-3 blockade with specific antitumor vaccination resulted in a significant increase in activated CD8+ T cells in the tumor and disruption of the tumor parenchyma. A major component of this effect was CD4 independent and required LAG-3 expression by CD8+ T cells. Taken together, these data demonstrate a direct role for LAG-3 on CD8+ T cells and suggest that LAG-3 blockade may be a potential cancer treatment.
Figures

References
-
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. . 2003;3:609–620. - PubMed
-
- Dong C., Nurieva R.I., Prasad D.V. Immune regulation by novel costimulatory molecules. Immunol. Res. . 2003;28:39–48. - PubMed
-
- Khoury S.J., Sayegh M.H. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. . 2004;20:529–538. - PubMed
-
- Wang S., Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. . 2004;6:759–766. - PubMed
-
- Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu. Rev. Immunol. . 2005;23:515–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

