Nonviral approaches for neuronal delivery of nucleic acids
- PMID: 17932730
- PMCID: PMC2292496
- DOI: 10.1007/s11095-007-9439-5
Nonviral approaches for neuronal delivery of nucleic acids
Abstract
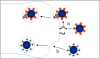
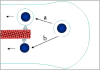
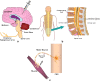
The delivery of therapeutic nucleic acids to neurons has the potential to treat neurological disease and spinal cord injury. While select viral vectors have shown promise as gene carriers to neurons, their potential as therapeutic agents is limited by their toxicity and immunogenicity, their broad tropism, and the cost of large-scale formulation. Nonviral vectors are an attractive alternative in that they offer improved safety profiles compared to viruses, are less expensive to produce, and can be targeted to specific neuronal subpopulations. However, most nonviral vectors suffer from significantly lower transfection efficiencies than neurotropic viruses, severely limiting their utility in neuron-targeted delivery applications. To realize the potential of nonviral delivery technology in neurons, vectors must be designed to overcome a series of extra- and intracellular barriers. In this article, we describe the challenges preventing successful nonviral delivery of nucleic acids to neurons and review strategies aimed at overcoming these challenges.
Figures



References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9126153', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9126153/'}]}
- W. J. Bowers, D. F. Howard, and H. J. Federoff. Gene therapeutic strategies for neuroprotection: implications for Parkinson’s disease. Exp. Neurol.144:58–68 (1997). - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16629633', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16629633/'}]}
- B. Blits, and M. B. Bunge. Direct gene therapy for repair of the spinal cord. J. Neurotrauma.23:508–520 (2006). - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11096437', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11096437/'}]}
- J. M. Alisky, and B. L. Davidson. Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum. Gene. Ther.11:2315–2329 (2000). - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '14653844', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14653844/'}]}
- R. Tinsley, and P. Eriksson. Use of gene therapy in central nervous system repair. Acta. Neurol. Scand.109:1–8 (2004). - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16228969', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16228969/'}]}
- T. Federici, and N. M. Boulis. Gene-based treatment of motor neuron diseases. Muscle Nerve.33:302–323 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

