Power-limited contraction dynamics of Vorticella convallaria: an ultrafast biological spring
- PMID: 17933875
- PMCID: PMC2134882
- DOI: 10.1529/biophysj.107.108852
Power-limited contraction dynamics of Vorticella convallaria: an ultrafast biological spring
Abstract
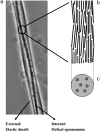
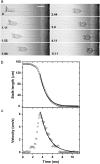
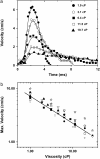
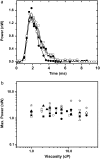
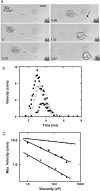
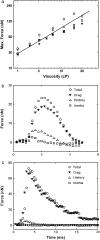
Vorticella convallaria is one of the fastest and most powerful cellular machines. The cell body is attached to a substrate by a slender stalk containing a polymeric structure-the spasmoneme. Helical coiling of the stalk results from rapid contraction of the spasmoneme, an event mediated by calcium binding to a negatively charged polymeric backbone. We use high speed imaging to measure the contraction velocity as a function of the viscosity of the external environment and find that the maximum velocity scales inversely with the square root of the viscosity. This can be explained if the rate of contraction is ultimately limited by the power delivered by the actively contracting spasmoneme. Microscopically, this scenario would arise if the mechanochemical wave that propagates along the spasmoneme is faster than the rate at which the cell body can respond due to its large hydrodynamic resistance. We corroborate this by using beads as markers on the stalk and find that the contraction starts at the cell body and proceeds down the stalk at a speed that exceeds the velocity of the cell body.
Figures
Comment in
-
Don'T blink: observing the ultra-fast contraction of spasmonemes.Biophys J. 2008 Jan 1;94(1):4-5. doi: 10.1529/biophysj.107.118166. Epub 2007 Oct 12. Biophys J. 2008. PMID: 17933874 Free PMC article. No abstract available.
References
-
- Katoh, K., and Y. Naitoh. 1992. A mechanosensory mechanism for evoking a cellular contraction in Vorticella. J. Exp. Biol. 168:253–267.
-
- Amos, W. B. 1975. Contraction and calcium binding in the Vorticellid ciliates. In Molecules and Cell Movement. S. Inoue and R. E. Stephens, editors. Raven Press, New York. 411–436. - PubMed
-
- Amos, W. B. 1972. Structure and coiling of the stalk in the peritrich ciliates Vorticella and Carchesium. J. Cell Sci. 10:95–122. - PubMed
-
- Maciejewski, J. J., E. J. Vacchiano, S. M. McCutcheon, and H. E. Buhse Jr. 1999. Cloning and expression of a cDNA encoding a Vorticella convallaria spasmin: an EF-hand calcium-binding protein. J. Eukaryot. Microbiol. 46:165–173. - PubMed
-
- Asai, H., T. Ninomiya, R. Kono, and Y. Moriyama. 1998. Spasmin and a putative spasmin binding protein(s) isolated from solubilized spasmonemes. J. Eukaryot. Microbiol. 45:33–39.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

