Investigating interactions mediated by the presynaptic protein bassoon in living cells by Foerster's resonance energy transfer and fluorescence lifetime imaging microscopy
- PMID: 17933880
- PMCID: PMC2212683
- DOI: 10.1529/biophysj.107.111674
Investigating interactions mediated by the presynaptic protein bassoon in living cells by Foerster's resonance energy transfer and fluorescence lifetime imaging microscopy
Abstract
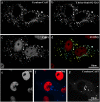
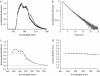
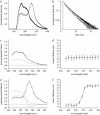
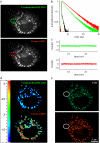
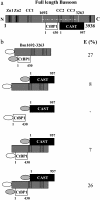
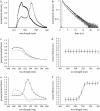
Neuronal synapses are highly specialized structures for communication between nerve cells. Knowledge about their molecular organization and dynamics is still incomplete. The large multidomain protein Bassoon plays a major role in scaffolding and organizing the cytomatrix at the active zone of neurotransmitter release in presynaptic boutons. Utilizing immunofluorescence techniques, we show that Bassoon is essential for corecruitment of its synaptic interaction partners, C-terminal binding protein 1/brefeldin A-dependent ADP-ribosylation substrate and CAZ-associated structural protein, into protein complexes upon heterologous expression in COS-7 cells. A combination of Foerster's resonance energy transfer and fluorescence lifetime imaging microscopy in the time domain was adopted to investigate the potential for the association of these proteins in the same complexes. A direct physical association between Bassoon and CtBP1 could also be observed at synapses of living hippocampal neurons. Simultaneous analysis of fluorescence decays of the donor and the acceptor probes along with their decay-associated spectra allowed a clear discrimination of energy transfer.
Figures



Similar articles
-
Regulation of presynaptic anchoring of the scaffold protein Bassoon by phosphorylation-dependent interaction with 14-3-3 adaptor proteins.PLoS One. 2013;8(3):e58814. doi: 10.1371/journal.pone.0058814. Epub 2013 Mar 14. PLoS One. 2013. PMID: 23516560 Free PMC article.
-
Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals.J Cell Biol. 1998 Jul 27;142(2):499-509. doi: 10.1083/jcb.142.2.499. J Cell Biol. 1998. PMID: 9679147 Free PMC article.
-
Molecular organization and assembly of the presynaptic active zone of neurotransmitter release.Results Probl Cell Differ. 2006;43:49-68. doi: 10.1007/400_012. Results Probl Cell Differ. 2006. PMID: 17068967 Review.
-
Functional regions of the presynaptic cytomatrix protein bassoon: significance for synaptic targeting and cytomatrix anchoring.Mol Cell Neurosci. 2003 Jun;23(2):279-91. doi: 10.1016/s1044-7431(03)00015-0. Mol Cell Neurosci. 2003. PMID: 12812759
-
Molecular organization of the presynaptic active zone.Cell Tissue Res. 2006 Nov;326(2):379-91. doi: 10.1007/s00441-006-0244-y. Epub 2006 Jul 25. Cell Tissue Res. 2006. PMID: 16865347 Review.
Cited by
-
Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone.Front Synaptic Neurosci. 2016 Jan 12;7:19. doi: 10.3389/fnsyn.2015.00019. eCollection 2015. Front Synaptic Neurosci. 2016. PMID: 26793095 Free PMC article. Review.
-
Vertebrate Presynaptic Active Zone Assembly: a Role Accomplished by Diverse Molecular and Cellular Mechanisms.Mol Neurobiol. 2018 Jun;55(6):4513-4528. doi: 10.1007/s12035-017-0661-9. Epub 2017 Jul 6. Mol Neurobiol. 2018. PMID: 28685386 Review.
-
Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6504-9. doi: 10.1073/pnas.1002307107. Epub 2010 Mar 23. Proc Natl Acad Sci U S A. 2010. PMID: 20332206 Free PMC article.
-
Trio, a Rho Family GEF, Interacts with the Presynaptic Active Zone Proteins Piccolo and Bassoon.PLoS One. 2016 Dec 1;11(12):e0167535. doi: 10.1371/journal.pone.0167535. eCollection 2016. PLoS One. 2016. PMID: 27907191 Free PMC article.
-
An evolutionarily conserved mechanism for presynaptic trapping.Cell Mol Life Sci. 2010 Jun;67(11):1751-4. doi: 10.1007/s00018-010-0343-5. Epub 2010 Mar 25. Cell Mol Life Sci. 2010. PMID: 20336344 Free PMC article. Review.
References
-
- Ballestrem, C., and B. Geiger. 2005. Application of microscope-based FRET to study molecular interactions in focal adhesions of live cells. Methods Mol. Biol. 294:321–334. - PubMed
-
- Jares-Erijman, E. A., and T. M. Jovin. 2003. FRET imaging. Nat. Biotechnol. 21:1387–1395. - PubMed
-
- Chen, Y., J. D. Mills, and A. Periasamy. 2003. Protein localization in living cells and tissues using FRET and FLIM. Differentiation. 71:528–541. - PubMed
-
- van Munster, E. B., and T. W. Gadella. 2005. Fluorescence lifetime imaging microscopy (FLIM). Adv. Biochem. Eng. Biotechnol. 95:143–175. - PubMed
-
- Nair, D. K., M. Jose, T. Kuner, W. Zuschratter, and R. Hartig. 2006. FRET-FLIM at nanometer spectral resolution from living cells. Opt. Express. 14:12217–12229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

