Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism
- PMID: 17933904
- PMCID: PMC2174704
- DOI: 10.1105/tpc.106.049817
Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism
Erratum in
- Plant Cell. 2007 Dec;19(12):4131-2
Abstract
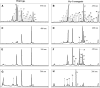
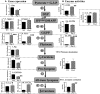
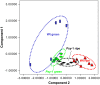
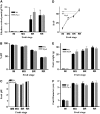
In tomato (Solanum lycopersicum), phytoene synthase-1 (PSY-1) is the key biosynthetic enzyme responsible for the synthesis of fruit carotenoids. To further our understanding of carotenoid formation in tomato fruit, we characterized the effect of constitutive expression of an additional tomato Psy-1 gene product. A quantitative data set defining levels of carotenoid/isoprenoid gene expression, enzyme activities, and metabolites was generated from fruit that showed the greatest perturbation in carotenoid content. Transcriptional upregulation, resulting in increased enzyme activities and metabolites, occurred only in the case of Psy-1, Psy-2, and lycopene cyclase B. For reactions involving 1-deoxy-d-xylulose5-phosphate synthase, geranylgeranyl diphosphate synthase, phytoene desaturase, zeta-carotene desaturase, carotene isomerase, and lycopene beta-cyclase, there were no correlations between gene expression, enzyme activities, and metabolites. Perturbations in carotenoid composition were associated with changes in plastid type and with chromoplast-like structures arising prematurely during fruit development. The levels of >120 known metabolites were determined. Comparison with the wild type illustrated that key metabolites (sucrose, glucose/fructose, and Glu) and sectors of intermediary metabolism (e.g., tricarboxylic [corrected] acid cycle intermediates and fatty acids) in the Psy-1 transgenic mature green fruit resembled changes in metabolism associated with fruit ripening. General fruit developmental and ripening properties, such as ethylene production and fruit firmness, were unaffected. Therefore, it appears that the changes to pigmentation, plastid type, and metabolism associated with Psy-1 overexpression are not connected with the ripening process.
Figures



Similar articles
-
Functional Validation of Phytoene Synthase and Lycopene ε-Cyclase Genes for High Lycopene Content in Autumn Olive Fruit (Elaeagnus umbellata).J Agric Food Chem. 2020 Oct 14;68(41):11503-11511. doi: 10.1021/acs.jafc.0c03092. Epub 2020 Sep 30. J Agric Food Chem. 2020. PMID: 32936623
-
Expression of carotenoid biosynthetic pathway genes and changes in carotenoids during ripening in tomato (Lycopersicon esculentum).Food Funct. 2011 Apr;2(3-4):168-73. doi: 10.1039/c0fo00169d. Epub 2011 Feb 18. Food Funct. 2011. PMID: 21779575
-
Phytoene synthase-2 enzyme activity in tomato does not contribute to carotenoid synthesis in ripening fruit.Plant Mol Biol. 1999 Jul;40(4):687-98. doi: 10.1023/a:1006256302570. Plant Mol Biol. 1999. PMID: 10480392
-
Regulation of carotenoid formation during tomato fruit ripening and development.J Exp Bot. 2002 Oct;53(377):2107-13. doi: 10.1093/jxb/erf059. J Exp Bot. 2002. PMID: 12324534 Review.
-
Regulation of carotenoid metabolism in tomato.Mol Plant. 2015 Jan;8(1):28-39. doi: 10.1016/j.molp.2014.11.006. Epub 2014 Dec 11. Mol Plant. 2015. PMID: 25578270 Review.
Cited by
-
Rapid identification of causal mutations in tomato EMS populations via mapping-by-sequencing.Nat Protoc. 2016 Dec;11(12):2401-2418. doi: 10.1038/nprot.2016.143. Epub 2016 Nov 3. Nat Protoc. 2016. PMID: 27809315
-
Transcriptome and Metabolite Profiling of Tomato SGR-Knockout Null Lines Using the CRISPR/Cas9 System.Int J Mol Sci. 2022 Dec 21;24(1):109. doi: 10.3390/ijms24010109. Int J Mol Sci. 2022. PMID: 36613549 Free PMC article.
-
Accumulation of carotenoids in Brassica rapa ssp. chinensis by a high proportion of blue in the light spectrum.Photochem Photobiol Sci. 2022 Nov;21(11):1947-1959. doi: 10.1007/s43630-022-00270-8. Epub 2022 Jul 27. Photochem Photobiol Sci. 2022. PMID: 35895283
-
Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening.Plant Cell. 2011 Mar;23(3):923-41. doi: 10.1105/tpc.110.081273. Epub 2011 Mar 11. Plant Cell. 2011. PMID: 21398570 Free PMC article.
-
Vitamin deficiencies in humans: can plant science help?Plant Cell. 2012 Feb;24(2):395-414. doi: 10.1105/tpc.111.093120. Epub 2012 Feb 28. Plant Cell. 2012. PMID: 22374394 Free PMC article. Review.
References
-
- Alexander, L., and Grierson, D. (2002). Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 53 2039–2055. - PubMed
-
- Bird, C.R., Smith, C.J.S., Ray, J.A., Moreau, P., Bevan, M.W., Bird, A.S., Hughes, S., Morris, P.C., Grierson, D., and Schuch, W. (1988). The tomato polygalacturonase gene and ripening-specific expression in transgenic plants. Plant Mol. Biol. 11 651–662. - PubMed
-
- Booker, J., Auldridge, M., Wills, S., McCarty, D., Klee, H., and Leyser, C. (2004). MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signalling molecule. Curr. Biol. 14 1232–1238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

