A Rho3 homolog is essential for appressorium development and pathogenicity of Magnaporthe grisea
- PMID: 17933908
- PMCID: PMC2168240
- DOI: 10.1128/EC.00104-07
A Rho3 homolog is essential for appressorium development and pathogenicity of Magnaporthe grisea
Abstract
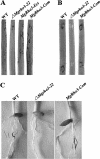
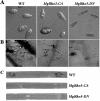
The small GTPase Rho3 is conserved in fungi and plays a key role in the control of cell polarity and exocytosis in yeast. In this report, we show that a Rho3 homolog, MgRho3, is dispensable for polarized hyphal growth in the rice blast fungus Magnaporthe grisea. However, MgRho3 is required for plant infection. Appressoria formed by the Mgrho3 deletion mutants are morphologically abnormal and defective in plant penetration. Conidia of the Mgrho3 deletion mutants are narrower than those of the wild-type strain and delayed in germination. Transformants expressing a dominant negative Mgrho3 allele exhibit similar phenotypes as the Mgrho3 deletion mutant, while transformants expressing a constitutively active allele of MgRho3 can produce normal conidia but remain defective in appressorium formation and plant infection. In contrast, overexpression of wild-type MgRho3 increases the infectivity of M. grisea. Our results reveal a new role for the conserved Rho3 as a critical regulator of developmental processes and pathogenicity of M. grisea.
Figures







Similar articles
-
A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea.Plant Cell. 2005 Apr;17(4):1317-29. doi: 10.1105/tpc.104.029116. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749760 Free PMC article.
-
Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea.Mol Plant Microbe Interact. 2004 May;17(5):547-56. doi: 10.1094/MPMI.2004.17.5.547. Mol Plant Microbe Interact. 2004. PMID: 15141959
-
Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea.Plant Cell. 2006 Oct;18(10):2822-35. doi: 10.1105/tpc.105.038422. Epub 2006 Oct 20. Plant Cell. 2006. PMID: 17056708 Free PMC article.
-
Extracellular matrix protein gene, EMP1, is required for appressorium formation and pathogenicity of the rice blast fungus, Magnaporthe grisea.Mol Cells. 2004 Feb 29;17(1):166-73. Mol Cells. 2004. PMID: 15055545
-
The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea.Biochem Soc Trans. 2005 Apr;33(Pt 2):384-8. doi: 10.1042/BST0330384. Biochem Soc Trans. 2005. PMID: 15787612 Review.
Cited by
-
Magnaporthe oryzae CK2 Accumulates in Nuclei, Nucleoli, at Septal Pores and Forms a Large Ring Structure in Appressoria, and Is Involved in Rice Blast Pathogenesis.Front Cell Infect Microbiol. 2019 Apr 17;9:113. doi: 10.3389/fcimb.2019.00113. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31058100 Free PMC article.
-
MoCps1 is important for conidiation, conidial morphology and virulence in Magnaporthe oryzae.Curr Genet. 2016 Nov;62(4):861-871. doi: 10.1007/s00294-016-0593-3. Epub 2016 Mar 15. Curr Genet. 2016. PMID: 26979515
-
Fsr1, a striatin homologue, forms an endomembrane-associated complex that regulates virulence in the maize pathogen Fusarium verticillioides.Mol Plant Pathol. 2018 Apr;19(4):812-826. doi: 10.1111/mpp.12562. Epub 2017 Jul 25. Mol Plant Pathol. 2018. PMID: 28467007 Free PMC article.
-
The Genomes of Three Uneven Siblings: Footprints of the Lifestyles of Three Trichoderma Species.Microbiol Mol Biol Rev. 2016 Feb 10;80(1):205-327. doi: 10.1128/MMBR.00040-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2016. PMID: 26864432 Free PMC article. Review.
-
Transcriptomic Dynamics of Active and Inactive States of Rho GTPase MoRho3 in Magnaporthe oryzae.J Fungi (Basel). 2022 Oct 11;8(10):1060. doi: 10.3390/jof8101060. J Fungi (Basel). 2022. PMID: 36294629 Free PMC article.
References
-
- Bourett, T. M., and R. J. Howard. 1992. Actin in penetration pegs of the fungal rice blast pathogen, Magnaporthe grisea. Protoplasma 168:20-26.
-
- Bourett, T. M., J. A. Sweigard, K. J. Czymmek, A. Carroll, and R. J. Howard. 2002. Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet. Biol. 37:211-220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

