Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction
- PMID: 17933909
- PMCID: PMC2168260
- DOI: 10.1128/EC.00350-07
Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction
Abstract
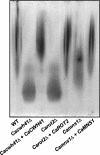
The cell surface of Candida albicans is enriched in highly glycosylated mannoproteins that are involved in the interaction with the host tissues. N glycosylation is a posttranslational modification that is initiated in the endoplasmic reticulum (ER), where the Glc(3)Man(9)GlcNAc(2) N-glycan is processed by alpha-glucosidases I and II and alpha1,2-mannosidase to generate Man(8)GlcNAc(2). This N-oligosaccharide is then elaborated in the Golgi to form N-glycans with highly branched outer chains rich in mannose. In Saccharomyces cerevisiae, CWH41, ROT2, and MNS1 encode for alpha-glucosidase I, alpha-glucosidase II catalytic subunit, and alpha1,2-mannosidase, respectively. We disrupted the C. albicans CWH41, ROT2, and MNS1 homologs to determine the importance of N-oligosaccharide processing on the N-glycan outer-chain elongation and the host-fungus interaction. Yeast cells of Cacwh41Delta, Carot2Delta, and Camns1Delta null mutants tended to aggregate, displayed reduced growth rates, had a lower content of cell wall phosphomannan and other changes in cell wall composition, underglycosylated beta-N-acetylhexosaminidase, and had a constitutively activated PKC-Mkc1 cell wall integrity pathway. They were also attenuated in virulence in a murine model of systemic infection and stimulated an altered pro- and anti-inflammatory cytokine profile from human monocytes. Therefore, N-oligosaccharide processing by ER glycosidases is required for cell wall integrity and for host-fungus interactions.
Figures



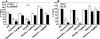
Similar articles
-
Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence.J Biol Chem. 2005 Jun 17;280(24):23408-15. doi: 10.1074/jbc.M502162200. Epub 2005 Apr 20. J Biol Chem. 2005. PMID: 15843378
-
Processing glycosidases of Saccharomyces cerevisiae.Biochim Biophys Acta. 1999 Jan 6;1426(2):275-85. doi: 10.1016/s0304-4165(98)00129-9. Biochim Biophys Acta. 1999. PMID: 9878780 Review.
-
Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans.J Biol Chem. 2006 Jan 6;281(1):90-8. doi: 10.1074/jbc.M510360200. Epub 2005 Nov 1. J Biol Chem. 2006. PMID: 16263704
-
Isolation and functional characterization of Sporothrix schenckii ROT2, the encoding gene for the endoplasmic reticulum glucosidase II.Fungal Biol. 2012 Aug;116(8):910-8. doi: 10.1016/j.funbio.2012.06.002. Epub 2012 Jun 28. Fungal Biol. 2012. PMID: 22862919
-
Importance of glycosidases in mammalian glycoprotein biosynthesis.Biochim Biophys Acta. 1999 Dec 6;1473(1):96-107. doi: 10.1016/s0304-4165(99)00171-3. Biochim Biophys Acta. 1999. PMID: 10580131 Review.
Cited by
-
Candida tropicalis PMT2 Is a Dispensable Gene for Viability but Required for Proper Interaction with the Host.J Fungi (Basel). 2024 Jul 20;10(7):502. doi: 10.3390/jof10070502. J Fungi (Basel). 2024. PMID: 39057387 Free PMC article.
-
Endoplasmic reticulum glucosidases and protein quality control factors cooperate to establish biotrophy in Ustilago maydis.Plant Cell. 2013 Nov;25(11):4676-90. doi: 10.1105/tpc.113.115691. Epub 2013 Nov 26. Plant Cell. 2013. PMID: 24280385 Free PMC article.
-
Influences of the Culturing Media in the Virulence and Cell Wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa.J Fungi (Basel). 2020 Nov 28;6(4):323. doi: 10.3390/jof6040323. J Fungi (Basel). 2020. PMID: 33260702 Free PMC article.
-
Functional analysis of Candida albicans GPI-anchored proteins: roles in cell wall integrity and caspofungin sensitivity.Fungal Genet Biol. 2008 Oct;45(10):1404-14. doi: 10.1016/j.fgb.2008.08.003. Epub 2008 Aug 15. Fungal Genet Biol. 2008. PMID: 18765290 Free PMC article.
-
Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1.PLoS Pathog. 2013;9(4):e1003315. doi: 10.1371/journal.ppat.1003315. Epub 2013 Apr 18. PLoS Pathog. 2013. PMID: 23637604 Free PMC article.
References
-
- Bates, S., D. M. MacCallum, G. Bertram, C. A. Munro, H. B. Hughes, E. T. Buurman, A. J. P. Brown, F. C. Odds, and N. A. R. Gow. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280:23408-23415. - PubMed
-
- Bates, S., H. B. Hughes, C. A. Munro, W. P. H. Thomas, D. M. MacCallum, G. Bertram, A. Atrih, M. A. J. Ferguson, A. J. P. Brown, F. C. Odds, and N. A. R. Gow. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90-98. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

