The visceral pericardium: macromolecular structure and contribution to passive mechanical properties of the left ventricle
- PMID: 17933976
- PMCID: PMC2878718
- DOI: 10.1152/ajpheart.00967.2007
The visceral pericardium: macromolecular structure and contribution to passive mechanical properties of the left ventricle
Abstract
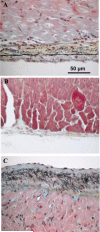
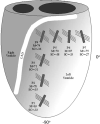
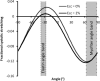
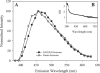
Much attention has been focused on the passive mechanical properties of the myocardium, which determines left ventricular (LV) diastolic mechanics, but the significance of the visceral pericardium (VP) has not been extensively studied. A unique en face three-dimensional volumetric view of the porcine VP was obtained using two-photon excitation fluorescence to detect elastin and backscattered second harmonic generation to detect collagen, in addition to standard light microscopy with histological staining. Below a layer of mesothelial cells, collagen and elastin fibers, extending several millimeters, form several distinct layers. The configuration of the collagen and elastin layers as well as the location of the VP at the epicardium providing a geometric advantage led to the hypothesis that VP mechanical properties play a role in the residual stress and passive stiffness of the heart. The removal of the VP by blunt dissection from porcine LV slices changed the opening angle from 53.3 +/- 10.3 to 27.3 +/- 5.7 degrees (means +/- SD, P < 0.05, n = 4). In four porcine hearts where the VP was surgically disrupted, a significant decrease in opening angle was found (35.5 +/- 4.0 degrees ) as well as a rightward shift in the ex vivo pressure-volume relationship before and after disruption and a decrease in LV passive stiffness at lower LV volumes (P < 0.05). These data demonstrate the significant and previously unreported role that the VP plays in the residual stress and passive stiffness of the heart. Alterations in this layer may occur in various disease states that effect diastolic function.
Figures





References
-
- Agostoni E, Bodega F, Zocchi L. Equivalent radius of paracellular “pores” of the mesothelium. J Appl Physiol. 1999;87:538–544. - PubMed
-
- Aletras AH, Balaban RS, Wen H. High-resolution strain analysis of the human heart with fast-DENSE. J Magn Reson. 1999;140:41–57. - PubMed
-
- Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res. 2000;87:235–240. - PubMed
-
- Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin. 2000;18:435–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

