Enantioselective Tsuji allylations
- PMID: 17935094
- PMCID: PMC2967289
- DOI: 10.1002/asia.200700183
Enantioselective Tsuji allylations
Abstract
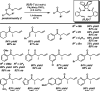
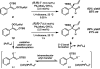
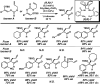
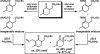
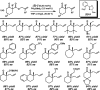
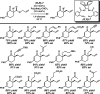
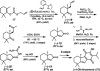
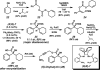
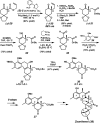
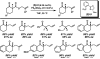
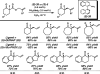
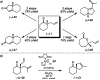
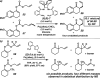
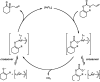
The family of allylation reactions developed by Tsuji in the 1980s are capable of generating tertiary and quaternary carbon stereocenters from several synthetic precursors. Despite the utility of these transformations, they have seen little use in the synthesis of natural products. Recently, the power of these reactions was significantly enhanced by the development of enantioselective versions of these transformations. Applications of these methods to the enantioselective syntheses of natural products and pharmaceutical compounds highlight the importance of these developments.
Figures


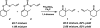





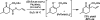
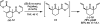
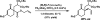



References
-
-
For excellent general reviews on the catalytic enantioselective generation of quaternary stereocenters, see: Trost BM, Jiang C. Synthesis. 2006:369–396. Christoffers J, Baro A, editors. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Wiley; Weinheim: 2005. Douglas CJ, Overman LE. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5363–5367. Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146. Christoffers J, Mann A. Angew. Chem. 2001;113:4725–4732. Angew. Chem. Int. Ed. 2001;40:4591–4597. Corey EJ, Guzman-Perez A. Angew. Chem. 1998;110:402–415. Angew. Chem. Int. Ed. 1998;37:388–401. Fuji K. Chem. Rev. 1993;93:2037–2066. Martin SF. Tetrahedron. 1980;36:419–460.
-
-
- Hayashi T, Kanehira K, Hagihara T, Kumada M. J. Org. Chem. 1988;53:113–120.
-
- Sawamura M, Nagata H, Sakamoto H, Ito Y. J. Am. Chem. Soc. 1992;114:2586–2592.
- Sawamura M, Sudoh M, Ito Y. J. Am. Chem. Soc. 1996;118:3309–3310.
- Kuwano R, Ito Y. J. Am. Chem. Soc. 1999;121:3236–3237.
- Kuwano R, Uchida K, Ito Y. Org. Lett. 2003;5:2177–2179. - PubMed
-
- You S-L, Hou X-L, Dai L-X, Cao B-X, Sun J. Chem. Commun. 2000:1933–1934.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

