Mechanism of Na(+) binding to thrombin resolved by ultra-rapid kinetics
- PMID: 17935858
- PMCID: PMC2111677
- DOI: 10.1016/j.bpc.2007.09.009
Mechanism of Na(+) binding to thrombin resolved by ultra-rapid kinetics
Abstract
The interaction of Na(+) and K(+) with proteins is at the basis of numerous processes of biological importance. However, measurement of the kinetic components of the interaction has eluded experimentalists for decades because the rate constants are too fast to resolve with conventional stopped-flow methods. Using a continuous-flow apparatus with a dead time of 50 micro s we have been able to resolve the kinetic rate constants and entire mechanism of Na(+) binding to thrombin, an interaction that is at the basis of the procoagulant and prothrombotic roles of the enzyme in the blood.
Figures
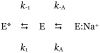


Similar articles
-
Rapid kinetics of Na+ binding to thrombin.J Biol Chem. 2006 Dec 29;281(52):40049-56. doi: 10.1074/jbc.M608600200. Epub 2006 Oct 30. J Biol Chem. 2006. PMID: 17074754
-
Thrombin allostery.Phys Chem Chem Phys. 2007 Mar 21;9(11):1291-306. doi: 10.1039/b616819a. Epub 2007 Jan 12. Phys Chem Chem Phys. 2007. PMID: 17347701 Review.
-
Mutant N143P reveals how Na+ activates thrombin.J Biol Chem. 2009 Dec 25;284(52):36175-36185. doi: 10.1074/jbc.M109.069500. Epub 2009 Oct 21. J Biol Chem. 2009. PMID: 19846563 Free PMC article.
-
Murine thrombin lacks Na+ activation but retains high catalytic activity.J Biol Chem. 2006 Mar 17;281(11):7183-8. doi: 10.1074/jbc.M512082200. Epub 2006 Jan 20. J Biol Chem. 2006. PMID: 16428384
-
How Na+ activates thrombin--a review of the functional and structural data.Biol Chem. 2008 Aug;389(8):1025-35. doi: 10.1515/BC.2008.113. Biol Chem. 2008. PMID: 18979627 Free PMC article. Review.
Cited by
-
Proton Bridging in Catalysis by and Inhibition of Serine Proteases of the Blood Cascade System.Life (Basel). 2021 Apr 27;11(5):396. doi: 10.3390/life11050396. Life (Basel). 2021. PMID: 33925363 Free PMC article. Review.
-
Sodium-induced population shift drives activation of thrombin.Sci Rep. 2020 Jan 23;10(1):1086. doi: 10.1038/s41598-020-57822-0. Sci Rep. 2020. PMID: 31974511 Free PMC article.
-
Analysis of adenosine A₂a receptor stability: effects of ligands and disulfide bonds.Biochemistry. 2010 Nov 2;49(43):9181-9. doi: 10.1021/bi101155r. Biochemistry. 2010. PMID: 20853839 Free PMC article.
-
Thrombin.Mol Aspects Med. 2008 Aug;29(4):203-54. doi: 10.1016/j.mam.2008.01.001. Epub 2008 Feb 1. Mol Aspects Med. 2008. PMID: 18329094 Free PMC article. Review.
-
Structural identification of the pathway of long-range communication in an allosteric enzyme.Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):1832-7. doi: 10.1073/pnas.0710894105. Epub 2008 Feb 4. Proc Natl Acad Sci U S A. 2008. PMID: 18250335 Free PMC article.
References
-
- Suelter CH. Enzymes activated by monovalent cations. Science. 1970;168:789–795. - PubMed
-
- Di Cera E. A structural perspective on enzymes activated by monovalent cations. J Biol Chem. 2006;281:1305–1308. - PubMed
-
- Page MJ, Di Cera E. Role of Na+ and K+ in enzyme function. Physiol Rev. 2006;86:1049–1092. - PubMed
-
- Di Cera E. Thrombin interactions. Chest. 2003;124:11S–17S. - PubMed
-
- Di Cera E, Page MJ, Bah A, Bush-Pelc LA, Garvey LC. Thrombin allostery. Phys Chem Chem Phys. 2007;9:1292–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

