A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy
- PMID: 17935893
- PMCID: PMC2897748
- DOI: 10.1016/j.neuroscience.2007.07.067
A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy
Abstract
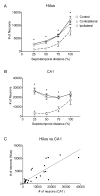
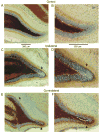
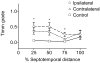
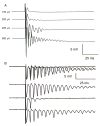
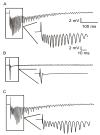
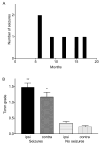
Ischemic brain injury is one of the leading causes of epilepsy in the elderly, and there are currently no adult rodent models of global ischemia, unilateral hemispheric ischemia, or focal ischemia that report the occurrence of spontaneous motor seizures following ischemic brain injury. The rodent hypoxic-ischemic (H-I) model of brain injury in adult rats is a model of unilateral hemispheric ischemic injury. Recent studies have shown that an H-I injury in perinatal rats causes hippocampal mossy fiber sprouting and epilepsy. These experiments aimed to test the hypothesis that a unilateral H-I injury leading to severe neuronal loss in young-adult rats also causes mossy fiber sprouting and spontaneous motor seizures many months after the injury, and that the mossy fiber sprouting induced by the H-I injury forms new functional recurrent excitatory synapses. The right common carotid artery of 30-day old rats was permanently ligated, and the rats were placed into a chamber with 8% oxygen for 30 min. A quantitative stereologic analysis revealed that the ipsilateral hippocampus had significant hilar and CA1 pyramidal neuronal loss compared with the contralateral and sham-control hippocampi. The septal region from the ipsilateral and contralateral hippocampus had small but significantly increased amounts of Timm staining in the inner molecular layer compared with the sham-control hippocampi. Three of 20 lesioned animals (15%) were observed to have at least one spontaneous motor seizure 6-12 months after treatment. Approximately 50% of the ipsilateral and contralateral hippocampal slices displayed abnormal electrophysiological responses in the dentate gyrus, manifest as all-or-none bursts to hilar stimulation. This study suggests that H-I injury is associated with synaptic reorganization in the lesioned region of the hippocampus, and that new recurrent excitatory circuits can predispose the hippocampus to abnormal electrophysiological activity and spontaneous motor seizures.
Figures



Similar articles
-
Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia.Epilepsia. 2004 Oct;45(10):1210-8. doi: 10.1111/j.0013-9580.2004.60403.x. Epilepsia. 2004. PMID: 15461675
-
Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy.J Comp Neurol. 2007 Dec 20;505(6):716-37. doi: 10.1002/cne.21533. J Comp Neurol. 2007. PMID: 17948865 Free PMC article.
-
Reassessment of the effects of cycloheximide on mossy fiber sprouting and epileptogenesis in the pilocarpine model of temporal lobe epilepsy.J Neurophysiol. 2002 Oct;88(4):2075-87. doi: 10.1152/jn.2002.88.4.2075. J Neurophysiol. 2002. PMID: 12364529
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
-
Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy.Epilepsia. 2012 Jun;53 Suppl 1:98-108. doi: 10.1111/j.1528-1167.2012.03481.x. Epilepsia. 2012. PMID: 22612814 Review.
Cited by
-
Primary and secondary mechanisms of epileptogenesis in the temporal lobe: there is a before and an after.Epilepsy Curr. 2010 Sep;10(5):118-25. doi: 10.1111/j.1535-7511.2010.01376.x. Epilepsy Curr. 2010. PMID: 20944823 Free PMC article.
-
ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat.Exp Neurol. 2010 Aug;224(2):369-88. doi: 10.1016/j.expneurol.2010.04.013. Epub 2010 Apr 24. Exp Neurol. 2010. PMID: 20420832 Free PMC article.
-
Immune Challenges and Seizures: How Do Early Life Insults Influence Epileptogenesis?Front Pharmacol. 2020 Feb 4;11:2. doi: 10.3389/fphar.2020.00002. eCollection 2020. Front Pharmacol. 2020. PMID: 32116690 Free PMC article. Review.
-
Neonatal Seizure Models to Study Epileptogenesis.Front Pharmacol. 2018 Apr 18;9:385. doi: 10.3389/fphar.2018.00385. eCollection 2018. Front Pharmacol. 2018. PMID: 29720941 Free PMC article. Review.
-
Optimized methods for epilepsy therapy development using an etiologically realistic model of focal epilepsy in the rat.Exp Neurol. 2015 Feb;264:150-62. doi: 10.1016/j.expneurol.2014.12.010. Epub 2014 Dec 16. Exp Neurol. 2015. PMID: 25523813 Free PMC article.
References
-
- Amaral DG. A golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. - PubMed
-
- Arabadzisz D, Freund TF. Changes in excitatory and inhibitory circuits of the rat hippocampus 12–14 months after complete forebrain ischemia. Neuroscience. 1999;92:27–45. - PubMed
-
- Arabadzisz D, Ylinen A, Emri Z. Increased inter-spike intervals and fast after-hyperpolarization of action potentials in rat hippocampal pyramidal cells accompanied with altered calbindin immunoreactivity 10–12 months after global forebrain ischemia. Neurosci Lett. 2002;331:103–106. - PubMed
-
- Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987. pp. 511–540.
-
- Berges S, Moulin Berger E, Tatu L, Sablot D, Challier B, Rumbach L. Seizures and epilepsy following strokes: recurrence factors. Eur Neurol. 2000;43:3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

