Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation
- PMID: 17936032
- PMCID: PMC2151979
- DOI: 10.1016/j.immuni.2007.08.015
Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation
Abstract
The maturation of dendritic cells (DCs) after exposure to microbial products or inflammatory mediators plays a critical role in initiating the immune response. We found that maturation can also occur under steady-state conditions, triggered by alterations in E-cadherin-mediated DC-DC adhesion. Selective disruption of these interactions induced the typical features of DC maturation including the upregulation of costimulatory molecules, MHC class II, and chemokine receptors. These events were triggered at least in part by activation of the beta-catenin pathway. However, unlike maturation induced by microbial products, E-cadherin-stimulated DCs failed to release immunostimulatory cytokines, exhibiting an entirely different transcriptional profile. As a result, E-cadherin-stimulated DCs elicited an entirely different T cell response in vivo, generating T cells with a regulatory as opposed to an effector phenotype. These DCs induced tolerance in vivo and may thus contribute to the elusive steady-state "tolerogenic DCs."
Figures
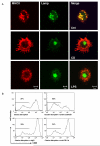

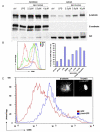
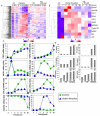
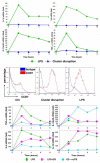
Comment in
-
Dendritic cells break bonds to tolerize.Immunity. 2007 Oct;27(4):544-6. doi: 10.1016/j.immuni.2007.10.004. Immunity. 2007. PMID: 17967408 Review.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. - PubMed
-
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leadsto antigen presentation on major histocompatibility complex class I products and peripheral CD8+ Tcell tolerance. J Exp Med. 2002;196:1627–1638. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

