Self-sacrifice in radical S-adenosylmethionine proteins
- PMID: 17936058
- PMCID: PMC2637762
- DOI: 10.1016/j.cbpa.2007.08.028
Self-sacrifice in radical S-adenosylmethionine proteins
Abstract
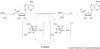
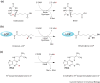
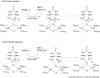
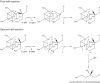
The radical SAM superfamily of metalloproteins catalyze the reductive cleavage of S-adenosyl-l-methionine to generate a 5'-deoxyadenosyl radical (5'-dA*) intermediate that is obligate for turnover. The 5'-dA* acts as a potent oxidant, initiating turnover by abstracting a hydrogen atom from an appropriate substrate. A special class of these enzymes use this strategy to functionalize unactivated C-H bonds by insertion of sulfur atoms. This review will describe the characterization of three members of this class - biotin synthase, lipoyl synthase, and MiaB protein - each of which has been shown to cannibalize itself during turnover.
Figures

References
-
- Frey PA, Hegeman AD, Reed GH. Free radical mechanisms in enzymology. Chem Rev. 2006;106:3302–3316. - PubMed
-
- Frey PA, Magnusson OT. S-Adenosylmethionine: a wolf in sheep's clothing, or a rich man's adenosylcobalamin? Chem Rev. 2003;103:2129–2148. - PubMed
-
- Frey PA. Importance of organic radicals in enzymic cleavage of unactivated carbon-hydrogen bonds. Chem Rev. 1990;90:1343–1357.
-
- Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001;29:1097–1106. - PMC - PubMed
-
This paper is the first detailed bioinformatics study that recognized that proteins that use S-adenosylmethionine as the source of a 5′-deoxyadenosyl radical constituted a superfamily.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

