Progesterone binding to the alpha1-subunit of the Na/K-ATPase on the cell surface: insights from computational modeling
- PMID: 17936318
- PMCID: PMC2275170
- DOI: 10.1016/j.steroids.2007.08.012
Progesterone binding to the alpha1-subunit of the Na/K-ATPase on the cell surface: insights from computational modeling
Abstract
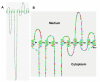
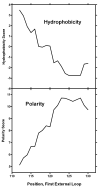
Progesterone triggers the resumption of meiosis in the amphibian oocyte through a signaling system at the plasma membrane. Analysis of [(3)H]ouabain and [(3)H]progesterone binding to the plasma membrane of the Rana pipiens oocyte indicates that progesterone competes with ouabain for a low affinity ouabain binding site on a 112kDa alpha1-subunit of the membrane Na/K-ATPase. Published amino acid sequences from both low and high affinity ouabain binding alpha1-subunits are compared, together with published site-directed mutagenesis studies of ouabain binding. We propose that the progesterone binding site is located in the external loop (23 amino acids) between the M1-M2 transmembrane helices. Analysis of loop topology and the countercurrent hydrophobicity/polarity gradients within the M1-M2 loop further suggest that the polar beta and hydrophobic alpha surfaces of the planar progesterone molecule interact with opposite sides of the amino acid loop. The 19-angular methyl group of progesterone is essential for activity; it could bind to the C-terminal region of the M1-M2 loop. Maximum biological activity requires formation of hydrogen-bond networks between the 3-keto group of progesterone and Arg(118), Asp(129) and possibly Glu(122-124) in the C-terminal region of the loop. The 20-keto group hydrogen may in turn hydrogen bond to Cys(111) near the M1 helix. Peptide flexibility undergoes a maximal transition near the midway point in the M1-M2 loop, suggesting that folding occurs within the loop, which further stabilizes progesterone binding.
Figures





Similar articles
-
Progesterone modulation of transmembrane helix-helix interactions between the alpha-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane.BMC Struct Biol. 2010 May 25;10:12. doi: 10.1186/1472-6807-10-12. BMC Struct Biol. 2010. PMID: 20500835 Free PMC article.
-
Caveolin-Na/K-ATPase interactions: role of transmembrane topology in non-genomic steroid signal transduction.Steroids. 2012 Sep;77(11):1160-8. doi: 10.1016/j.steroids.2012.04.012. Epub 2012 May 9. Steroids. 2012. PMID: 22579740
-
The steroid-binding subunit of the Na/K-ATPase as a progesterone receptor on the amphibian oocyte plasma membrane.Steroids. 2005 Dec 15;70(14):933-45. doi: 10.1016/j.steroids.2005.07.002. Epub 2005 Sep 13. Steroids. 2005. PMID: 16165176
-
Isoform-specific effects of charged residues at borders of the M1-M2 loop of the Na,K-ATPase alpha subunit.Biochemistry. 1999 Feb 23;38(8):2494-505. doi: 10.1021/bi982180j. Biochemistry. 1999. PMID: 10029544
-
Structure-function relationships of Na(+), K(+), ATP, or Mg(2+) binding and energy transduction in Na,K-ATPase.Biochim Biophys Acta. 2001 May 1;1505(1):57-74. doi: 10.1016/s0005-2728(00)00277-2. Biochim Biophys Acta. 2001. PMID: 11248189 Review.
Cited by
-
Glutamate Transporters/Na(+), K(+)-ATPase Involving in the Neuroprotective Effect as a Potential Regulatory Target of Glutamate Uptake.Mol Neurobiol. 2016 Mar;53(2):1124-1131. doi: 10.1007/s12035-014-9071-4. Epub 2015 Jan 14. Mol Neurobiol. 2016. PMID: 25586061 Review.
-
Gender differences in the development of uremic cardiomyopathy following partial nephrectomy: Role of progesterone.J Hypertens (Los Angel). 2013 Jan 31;2:10.4172/2167-1095.1000109. doi: 10.4172/2167-1095.1000109. J Hypertens (Los Angel). 2013. PMID: 24404431 Free PMC article.
-
Progesterone modulation of transmembrane helix-helix interactions between the alpha-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane.BMC Struct Biol. 2010 May 25;10:12. doi: 10.1186/1472-6807-10-12. BMC Struct Biol. 2010. PMID: 20500835 Free PMC article.
-
Tunable recognition of the steroid alpha-face by adjacent pi-electron density.Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13216-21. doi: 10.1073/pnas.0915142107. Epub 2010 Jul 12. Proc Natl Acad Sci U S A. 2010. PMID: 20624985 Free PMC article.
-
Plasma level of the endogenous sodium pump ligand marinobufagenin is related to the salt-sensitivity in men.J Hypertens. 2015 Mar;33(3):534-41; discussion 541. doi: 10.1097/HJH.0000000000000437. J Hypertens. 2015. PMID: 25479026 Free PMC article.
References
-
- Li X, Lonard DM, O'Malley BW. A contemporary understanding of progesterone receptor function. Mech. Ageing Dev. 2004;125:669–78. - PubMed
-
- Morrill GA, Kostellow AB. Progesterone induces meiotic division in the amphibian oocyte by releasing lipid second messengers from the plasma membrane. Steroids. 1999;64:157–67. - PubMed
-
- Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci. STKE. 2002:re9. 2002. - PubMed
-
- Smith LD, Ecker RE. Role of the oocyte nucleus in physiological maturation in Rana pipiens. Dev Biol. 1969;19:281–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

