Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation
- PMID: 17936705
- PMCID: PMC2083558
- DOI: 10.1016/j.molcel.2007.07.027
Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation
Abstract
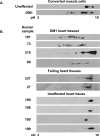
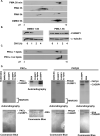
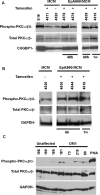
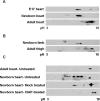
The genetic basis of myotonic dystrophy type 1 (DM1) is a CTG expansion in the 3' untranslated region (UTR) of DMPK. The pathogenic mechanism involves an RNA gain of function in which the repeat-containing transcripts accumulate in nuclei and alter the functions of RNA-binding proteins such as CUG-binding protein 1 (CUGBP1). CUGBP1 levels are increased in DM1 myoblasts, heart, and skeletal muscle tissues and in some DM1 mouse models. However, the molecular mechanisms for increased CUGBP1 in DM1 are unclear. Here, we demonstrate that expression of DMPK-CUG-repeat RNA results in hyperphosphorylation and stabilization of CUGBP1. CUGBP1 is hyperphosphorylated in DM1 tissues, cells, and a DM1 mouse model. Activation of PKC is required for CUGBP1 hyperphosphorylation in DM1 cells, and PKCalpha and betaII directly phosphorylate CUGBP1 in vitro. These results indicate that inappropriate activation of the PKC pathway contributes to the pathogenic effects of a noncoding RNA.
Figures



References
-
- Cardani R, Mancinelli E, Rotondo G, Sansone V, Meola G. Muscleblind-like protein 1 nuclear sequestration is a molecular pathology marker of DM1 and DM2. Eur J Histochem. 2006;50:177–182. - PubMed
-
- Charlet-B. N, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. - PubMed
-
- Dansithong W, Paul S, Comai L, Reddy S. MBNL1 is the primary determinant of focus formation and aberrant insulin receptor splicing in DM1. J Biol Chem. 2005;280:5773–5780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

