Generation and maintenance of Listeria-specific CD8+ T cell responses in perforin-deficient mice chronically infected with LCMV
- PMID: 17936870
- PMCID: PMC2214839
- DOI: 10.1016/j.virol.2007.08.038
Generation and maintenance of Listeria-specific CD8+ T cell responses in perforin-deficient mice chronically infected with LCMV
Abstract
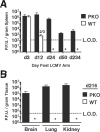
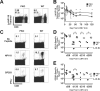
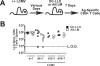
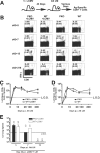
Disruption of the perforin gene results in primary immunodeficiency and an increased susceptibility to opportunistic pathogens. Perforin-deficient (PKO) mice fail to clear primary lymphocytic choriomeningitis virus (LCMV) Armstrong, resulting in persistent infection and functional exhaustion of virus-specific CD8+ T cells. CD8+ T cell responses to Listeria monocytogenes (LM) challenge within the first week after LCMV infection were diminished in both WT and PKO mice, and correlated with enhanced bacterial clearance. However, bacterial challenge at later time points generated similar CD8 T cell responses in both groups of mice. The phenotype and function of pre-existing LM-specific memory CD8+ T cells were maintained in persistently infected PKO mice. Thus persistent LCMV infection, as a result of perforin deficiency, results in dysfunction of the virus-specific CD8+ T cell response but does not compromise the host's ability to maintain pre-existing memory CD8+ T cells or to generate new memory CD8+ T cell responses against other pathogens.
Figures



Similar articles
-
Epitope specificity of memory CD8+ T cells dictates vaccination-induced mortality in LCMV-infected perforin-deficient mice.Eur J Immunol. 2012 Jun;42(6):1488-99. doi: 10.1002/eji.201142263. Eur J Immunol. 2012. PMID: 22678903 Free PMC article.
-
CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection.Infect Immun. 2002 Jan;70(1):153-62. doi: 10.1128/IAI.70.1.153-162.2002. Infect Immun. 2002. PMID: 11748177 Free PMC article.
-
Gammaherpesvirus latency differentially impacts the generation of primary versus secondary memory CD8+ T cells during subsequent infection.J Virol. 2014 Nov;88(21):12740-51. doi: 10.1128/JVI.02106-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142586 Free PMC article.
-
Deficient anti-listerial immunity in the absence of perforin can be restored by increasing memory CD8+ T cell numbers.J Immunol. 2003 Oct 15;171(8):4254-62. doi: 10.4049/jimmunol.171.8.4254. J Immunol. 2003. PMID: 14530349
-
Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma.J Immunol. 2000 Jun 15;164(12):6444-52. doi: 10.4049/jimmunol.164.12.6444. J Immunol. 2000. PMID: 10843700
Cited by
-
Chronic Toxoplasma gondii Infection Exacerbates Secondary Polymicrobial Sepsis.Front Cell Infect Microbiol. 2017 Apr 7;7:116. doi: 10.3389/fcimb.2017.00116. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28439500 Free PMC article.
-
Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection.Cell Host Microbe. 2010 Feb 18;7(2):103-14. doi: 10.1016/j.chom.2010.01.010. Cell Host Microbe. 2010. PMID: 20159617 Free PMC article.
-
CD8(+) T cells specific to a single Yersinia pseudotuberculosis epitope restrict bacterial replication in the liver but fail to provide sterilizing immunity.Infect Genet Evol. 2016 Sep;43:289-96. doi: 10.1016/j.meegid.2016.06.008. Epub 2016 Jun 4. Infect Genet Evol. 2016. PMID: 27268148 Free PMC article.
References
-
- Almousa H, Ouachee-Chardin M, Picard C, Radford-Weiss I, Caillat-Zucman S, Cavazzana-Calvo M, Blanche S, de Saint Basile G, Le Deist F, Fischer A. Transient familial haemophagocytic lymphohistiocytosis reactivation post-CD34 haematopoietic stem cell transplantation. Br J Haematol. 2005;130(3):404–8. - PubMed
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–6. - PubMed
-
- Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18(4):463–74. - PubMed
-
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 2002;3(7):619–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

