Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum
- PMID: 17937819
- PMCID: PMC2170451
- DOI: 10.1186/1742-4690-4-75
Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum
Abstract
Background: HIV-1 Vpu targets newly synthesized CD4 receptor for rapid degradation by a process reminiscent of endoplasmic reticulum (ER)-associated protein degradation (ERAD). Vpu is thought to act as an adaptor protein, connecting CD4 to the ubiquitin (Ub)-proteasome degradative system through an interaction with beta-TrCP, a component of the SCFbeta-TrCP E3 Ub ligase complex.
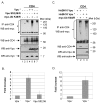
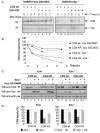
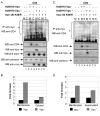
Results: Here, we provide direct evidence indicating that Vpu promotes trans-ubiquitination of CD4 through recruitment of SCFbeta-TrCP in human cells. To examine whether Ub conjugation occurs on the cytosolic tail of CD4, we substituted all four Ub acceptor lysine residues for arginines. Replacement of cytosolic lysine residues reduced but did not prevent Vpu-mediated CD4 degradation and ubiquitination, suggesting that Vpu-mediated CD4 degradation is not entirely dependent on the ubiquitination of cytosolic lysines and as such might also involve ubiquitination of other sites. Cell fractionation studies revealed that Vpu enhanced the levels of ubiquitinated forms of CD4 detected in association with not only the ER membrane but also the cytosol. Interestingly, significant amounts of membrane-associated ubiquitinated CD4 appeared to be fully dislocated since they could be recovered following sodium carbonate salt treatment. Finally, expression of a transdominant negative mutant of the AAA ATPase Cdc48/p97 involved in the extraction of ERAD substrates from the ER membrane inhibited Vpu-mediated CD4 degradation.
Conclusion: Taken together, these results are consistent with a model whereby HIV-1 Vpu targets CD4 for degradation by an ERAD-like process involving most likely poly-ubiquitination of the CD4 cytosolic tail by SCFbeta-TrCP prior to dislocation of receptor molecules across the ER membrane by a process that depends on the AAA ATPase Cdc48/p97.
Figures

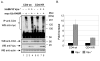
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

