The PhoQ-activating potential of antimicrobial peptides contributes to antimicrobial efficacy and is predictive of the induction of bacterial resistance
- PMID: 17938183
- PMCID: PMC2168022
- DOI: 10.1128/AAC.00854-07
The PhoQ-activating potential of antimicrobial peptides contributes to antimicrobial efficacy and is predictive of the induction of bacterial resistance
Abstract
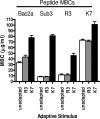
Antimicrobial peptides (AMPs) are among the leading candidates to replace antibiotics which have been rendered ineffective by the evolution of resistant bacterial strains. Concerns do exist, however, that the therapeutic administration of AMPs may also select for resistant strains but with much more dire consequences, as these peptides represent an endogenous and essential component of host immune defense. The recent demonstration that AMPs function as ligands for the bacterial sensory kinase PhoQ for the initiation of virulence and adaptive responses lends credence to these concerns. While the ability to serve as PhoQ ligands suggests that the therapeutic administration of AMPs could (i) exacerbate infections by promoting bacterial virulence and (ii) select resistant mutants by encouraging adaptive behaviors, it also provides a rational basis for AMP selection and optimization. Here, we demonstrate that derivatives of a representative AMP have differential abilities to serve as PhoQ ligands and that this correlates with the ability to induce bacterial adaptive responses. We propose that PhoQ-activating potential is a logical parameter for AMP optimization and introduce a novel strategy for the treatment of minimal bactericidal concentration data that permits the discrimination and quantification of the contributions of PhoQ-activating potential and direct antimicrobial activity to net antimicrobial efficiency.
Figures



Similar articles
-
Effect of PhoP-PhoQ activation by broad repertoire of antimicrobial peptides on bacterial resistance.J Biol Chem. 2012 Feb 10;287(7):4544-51. doi: 10.1074/jbc.M111.278523. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158870 Free PMC article.
-
Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance.Curr Med Chem. 2017;24(38):4303-4314. doi: 10.2174/0929867324666170815102441. Curr Med Chem. 2017. PMID: 28814242 Review.
-
Role of Pseudomonas aeruginosa PhoP-phoQ in resistance to antimicrobial cationic peptides and aminoglycosides.Microbiology (Reading). 2000 Oct;146 ( Pt 10):2543-2554. doi: 10.1099/00221287-146-10-2543. Microbiology (Reading). 2000. PMID: 11021929
-
Bacterial resistance to antimicrobial peptides.J Pept Sci. 2019 Nov;25(11):e3210. doi: 10.1002/psc.3210. Epub 2019 Oct 21. J Pept Sci. 2019. PMID: 31637796 Review.
-
Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa.Mol Microbiol. 2003 Oct;50(1):205-17. doi: 10.1046/j.1365-2958.2003.03673.x. Mol Microbiol. 2003. PMID: 14507375
Cited by
-
Cys-scanning disulfide crosslinking and bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ.Structure. 2014 Sep 2;22(9):1239-1251. doi: 10.1016/j.str.2014.04.019. Epub 2014 Jul 31. Structure. 2014. PMID: 25087511 Free PMC article.
-
An E. coli display method for characterization of peptide-sensor kinase interactions.Nat Chem Biol. 2023 Apr;19(4):451-459. doi: 10.1038/s41589-022-01207-z. Epub 2022 Dec 8. Nat Chem Biol. 2023. PMID: 36482094 Free PMC article.
-
Effect of PhoP-PhoQ activation by broad repertoire of antimicrobial peptides on bacterial resistance.J Biol Chem. 2012 Feb 10;287(7):4544-51. doi: 10.1074/jbc.M111.278523. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158870 Free PMC article.
-
How the PhoP/PhoQ System Controls Virulence and Mg2+ Homeostasis: Lessons in Signal Transduction, Pathogenesis, Physiology, and Evolution.Microbiol Mol Biol Rev. 2021 Aug 18;85(3):e0017620. doi: 10.1128/MMBR.00176-20. Epub 2021 Jun 30. Microbiol Mol Biol Rev. 2021. PMID: 34191587 Free PMC article. Review.
-
Racing on the Wrong Track.Front Chem. 2017 Jun 19;5:42. doi: 10.3389/fchem.2017.00042. eCollection 2017. Front Chem. 2017. PMID: 28674690 Free PMC article.
References
-
- Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. - PubMed
-
- Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. L. Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461-472. - PubMed
-
- Bell, G., and P. H. Gouyon. 2003. Arming the enemy: the evolution of resistance to self-proteins. Microbiology 149:1367-1375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

