Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology
- PMID: 17938190
- PMCID: PMC2223901
- DOI: 10.1128/AAC.00609-07
Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology
Abstract
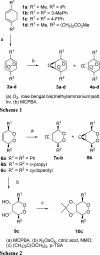
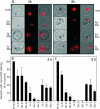
Artermisinin and its derivatives are now the mainstays of antimalarial treatment; however, their mechanism of action is only poorly understood. We report on the synthesis of a novel series of epoxy-endoperoxides that can be prepared in high yields from simple starting materials. Endoperoxides that are disubstituted with alkyl or benzyl side chains show efficient inhibition of the growth of both chloroquine-sensitive and -resistant strains of Plasmodium falciparum. A trans-epoxide with respect to the peroxide linkage increases the activity compared to that of its cis-epoxy counterpart or the parent endoperoxide. The novel endoperoxides do not show a strong interaction with artemisinin. We have compared the mechanism of action of the novel endoperoxides with that of artemisinin. Electron microscopy reveals that the novel endoperoxides cause the early accumulation of endocytic vesicles, while artemisinin causes the disruption of the digestive vacuole membrane. At longer incubation times artemisinin causes extensive loss of organellar structures, while the novel endoperoxides cause myelin body formation as well as the accumulation of endocytic vesicles. An early event following endoperoxide treatment is the redistribution of the pH-sensitive probe LysoSensor Blue from the digestive vacuole to punctate structures. By contrast, neither artemisinin nor the novel endoperoxides caused alterations in the morphology of the endoplasmic reticulum nor showed antagonistic antimalarial activity when they were used with thapsigargin. Analysis of rhodamine 123 uptake by P. falciparum suggests that disruption of the mitochondrial membrane potential occurs as a downstream effect rather than as an initiator of parasite killing. The data suggest that the digestive vacuole is an important initial site of endoperoxide antimalarial activity.
Figures





References
-
- Adovelande, J., J. Deleze, and J. Schrevel. 1998. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem. Pharmacol. 55:433-440. - PubMed
-
- Afonso, A., P. Hunt, S. Cheesman, A. C. Alves, C. V. Cunha, V. do Rosario, and P. Cravo. 2006. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 50:480-489. - PMC - PubMed
-
- Aley, S. B., J. A. Sherwood, K. Marsh, O. Eidelman, and R. J. Howard. 1986. Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology 92:511-525. - PubMed
-
- Arrow, K. J., C. B. Panosian, and H. Gelbrand. 2004. Saving lives, buying time: economics of malaria drugs in an age of resistance. The National Academies Press, Washington, DC. - PubMed
-
- Avery, M. A., W. K. M. Chong, and C. Jenning-White. 1992. Stereroselective total synthesis of (+)-artemisinin, the antimalarial constituent of Artemisia annua L. J. Am. Chem. Soc. 114:974-979.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

