c-Cbl-mediated regulation of LAT-nucleated signaling complexes
- PMID: 17938199
- PMCID: PMC2169400
- DOI: 10.1128/MCB.00467-07
c-Cbl-mediated regulation of LAT-nucleated signaling complexes
Abstract
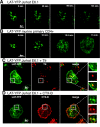
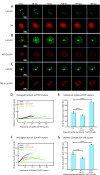
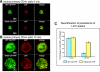
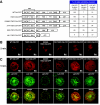
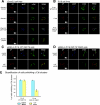
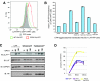
The engagement of the T-cell receptor (TCR) causes the rapid recruitment of multiple signaling molecules into clusters with the TCR. Upon receptor activation, the adapters LAT and SLP-76, visualized as chimeric proteins tagged with yellow fluorescent protein, transiently associate with and then rapidly dissociate from the TCR. Previously, we demonstrated that after recruitment into signaling clusters, SLP-76 is endocytosed in vesicles via a lipid raft-dependent pathway that requires the interaction of the endocytic machinery with ubiquitylated proteins. In this study, we focus on LAT and demonstrate that signaling clusters containing this adapter are internalized into distinct intracellular compartments and dissipate rapidly upon TCR activation. The internalization of LAT was inhibited in cells expressing versions of the ubiquitin ligase c-Cbl mutated in the RING domain and in T cells from mice lacking c-Cbl. Moreover, c-Cbl RING mutant forms suppressed LAT ubiquitylation and caused an increase in cellular LAT levels, as well as basal and TCR-induced levels of phosphorylated LAT. Collectively, these data indicate that following the rapid formation of signaling complexes upon TCR stimulation, c-Cbl activity is involved in the internalization and possible downregulation of a subset of activated signaling molecules.
Figures

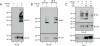
References
-
- Aguilar, R. C., and B. Wendland. 2003. Ubiquitin: not just for proteasomes anymore. Curr. Opin. Cell Biol. 15:184-190. - PubMed
-
- Alarcon, B., and M. Fresno. 1998. Transferrin receptor (CD71), p. 2389-2392. In P. J. Delves and I. M. Roitt (ed.), Encyclopedia of immunology, 2nd ed. Academic Press, London, United Kingdom.
-
- Barda-Saad, M., A. Braiman, R. Titerence, S. C. Bunnell, V. A. Barr, and L. E. Samelson. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6:80-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
