The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity
- PMID: 17938206
- PMCID: PMC2169424
- DOI: 10.1128/MCB.00157-07
The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity
Abstract
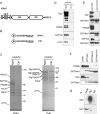
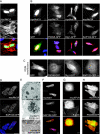
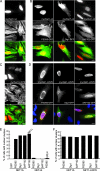
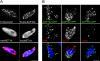
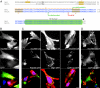
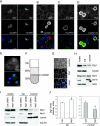
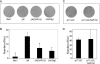
Net1 is a RhoA-specific guanine nucleotide exchange factor which localizes to the nucleus at steady state. A deletion in its N terminus redistributes the protein to the cytosol, where it activates RhoA and can promote transformation. Net1 contains a PDZ-binding motif at the C terminus which is essential for its transformation properties. Here, we found that Net1 interacts through its PDZ-binding motif with tumor suppressor proteins of the Dlg family, including Dlg1/SAP97, SAP102, and PSD95. The interaction between Net1 and its PDZ partners promotes the translocation of the PDZ proteins to nuclear subdomains associated with PML bodies. Interestingly, the oncogenic mutant of Net1 is unable to shuttle the PDZ proteins to the nucleus, although these proteins still associate as clusters in the cytosol. Our results suggest that the ability of oncogenic Net1 to transform cells may be in part related to its ability to sequester tumor suppressor proteins like Dlg1 in the cytosol, thereby interfering with their normal cellular function. In agreement with this, the transformation potential of oncogenic Net1 is reduced when it is coexpressed with Dlg1 or SAP102. Together, our results suggest that the interaction between Net1 and Dlg1 may contribute to the mechanism of Net1-mediated transformation.
Figures

Similar articles
-
Interaction of the RhoA exchange factor Net1 with discs large homolog 1 protects it from proteasome-mediated degradation and potentiates Net1 activity.J Biol Chem. 2009 Sep 4;284(36):24269-80. doi: 10.1074/jbc.M109.029439. Epub 2009 Jul 8. J Biol Chem. 2009. PMID: 19586902 Free PMC article.
-
Acetylation of the RhoA GEF Net1A controls its subcellular localization and activity.J Cell Sci. 2015 Mar 1;128(5):913-22. doi: 10.1242/jcs.158121. Epub 2015 Jan 14. J Cell Sci. 2015. PMID: 25588829 Free PMC article.
-
Contributions of the RhoA guanine nucleotide exchange factor Net1 to polyoma middle T antigen-mediated mammary gland tumorigenesis and metastasis.Breast Cancer Res. 2018 May 16;20(1):41. doi: 10.1186/s13058-018-0966-2. Breast Cancer Res. 2018. PMID: 29769144 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Gender differences in the context of interventions for improving health literacy in migrants: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Dec 12;12(12):CD013302. doi: 10.1002/14651858.CD013302.pub2. Cochrane Database Syst Rev. 2024. PMID: 39665382
Cited by
-
Expression of neuroepithelial transforming gene 1 is enhanced in oesophageal cancer and mediates an invasive tumour cell phenotype.J Exp Clin Cancer Res. 2013 Aug 14;32(1):55. doi: 10.1186/1756-9966-32-55. J Exp Clin Cancer Res. 2013. PMID: 23945136 Free PMC article.
-
Interaction of the RhoA exchange factor Net1 with discs large homolog 1 protects it from proteasome-mediated degradation and potentiates Net1 activity.J Biol Chem. 2009 Sep 4;284(36):24269-80. doi: 10.1074/jbc.M109.029439. Epub 2009 Jul 8. J Biol Chem. 2009. PMID: 19586902 Free PMC article.
-
mDia2 and CXCL12/CXCR4 chemokine signaling intersect to drive tumor cell amoeboid morphological transitions.Biochem Biophys Res Commun. 2017 Mar 4;484(2):255-261. doi: 10.1016/j.bbrc.2017.01.087. Epub 2017 Jan 21. Biochem Biophys Res Commun. 2017. PMID: 28115158 Free PMC article.
-
Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective.J Mammary Gland Biol Neoplasia. 2010 Mar;15(1):49-63. doi: 10.1007/s10911-010-9168-y. Epub 2010 Jan 27. J Mammary Gland Biol Neoplasia. 2010. PMID: 20101444 Free PMC article. Review.
-
Stress-activated MAPKs and CRM1 regulate the subcellular localization of Net1A to control cell motility and invasion.J Cell Sci. 2018 Feb 1;131(3):jcs204644. doi: 10.1242/jcs.204644. J Cell Sci. 2018. PMID: 29361525 Free PMC article.
References
-
- Alberts, A. S., H. Qin, H. S. Carr, and J. A. Frost. 2005. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J. Biol. Chem. 280:12152-12161. - PubMed
-
- Arthur, W. T., S. M. Ellerbroek, C. J. Der, K. Burridge, and K. Wennerberg. 2002. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J. Biol. Chem. 277:42964-42972. - PubMed
-
- Audebert, S., C. Navarro, C. Nourry, S. Chasserot-Golaz, P. Lecine, Y. Bellaiche, J. L. Dupont, R. T. Premont, C. Sempere, J. M. Strub, A. Van Dorsselaer, N. Vitale, and J. P. Borg. 2004. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14:987-995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
