The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42
- PMID: 17938223
- PMCID: PMC2223653
- DOI: 10.1128/IAI.01913-06
The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42
Abstract
Mycobacterium avium subsp. paratuberculosis infection of cattle takes place through the intestinal mucosa. To identify M. avium subsp. paratuberculosis genes associated with the invasion of bovine epithelial cells in vitro, we screened a library of transposon mutants. Several mutants of M. avium subsp. paratuberculosis were identified which invaded Madin-Darby bovine kidney (MDBK) epithelial cells less efficiently than wild-type (wt) M. avium subsp. paratuberculosis. The deltaOx mutant had the transposon located in the MAP3464 gene, a putative oxidoreductase gene whose expression is upregulated upon bacterial contact with MDBK cells. Complete restoration of invasion comparable to that for the wt bacterium was achieved by introducing a copy of the complete oxidoreductase operon into the deltaOx mutant. Immunoprecipitation and Western blot analysis indicated that wt M. avium subsp. paratuberculosis activates Cdc42 and RhoA pathways of internalization 15 and 60 min after infection of the host cell, respectively. The deltaOx mutant, however, failed to activate the Cdc42 pathway. To determine whether an M. avium subsp. paratuberculosis protein delivered to the host cell mediates the entry of the wt bacterium by activation of the Cdc42 pathway, affinity precipitation of active Cdc42 from MDBK-infected cells followed by mass spectrometry was carried out. We identified a 17-amino-acid bacterial peptide associated with the Cdc42 of cells infected with wt M. avium subsp. paratuberculosis but not with the deltaOx mutant. The sequence of the peptide matches MAP3985c, a hypothetical protein, possibly functioning as a putative Cdc42 effector. These findings reveal a novel signaling pathway activated during M. avium subsp. paratuberculosis entry that links the product of MAP3464 gene to activation of Cdc42 in the host cell.
Figures
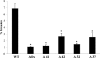



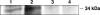


Similar articles
-
The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells.Infect Immun. 2006 May;74(5):2849-55. doi: 10.1128/IAI.74.5.2849-2855.2006. Infect Immun. 2006. PMID: 16622223 Free PMC article.
-
No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis.Infect Immun. 2013 Nov;81(11):3960-5. doi: 10.1128/IAI.00575-13. Epub 2013 Aug 12. Infect Immun. 2013. PMID: 23940208 Free PMC article. Review.
-
The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells.Microbiology (Reading). 2003 Aug;149(Pt 8):2061-2069. doi: 10.1099/mic.0.26323-0. Microbiology (Reading). 2003. PMID: 12904546
-
A 38-kilobase pathogenicity island specific for Mycobacterium avium subsp. paratuberculosis encodes cell surface proteins expressed in the host.Infect Immun. 2004 Mar;72(3):1265-74. doi: 10.1128/IAI.72.3.1265-1274.2004. Infect Immun. 2004. PMID: 14977927 Free PMC article.
-
Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis.Infect Immun. 2001 Apr;69(4):2075-82. doi: 10.1128/IAI.69.4.2075-2082.2001. Infect Immun. 2001. PMID: 11254560 Free PMC article.
Cited by
-
Delineating transcriptional crosstalk between Mycobacterium avium subsp. paratuberculosis and human THP-1 cells at the early stage of infection via dual RNA-seq analysis.Vet Res. 2022 Sep 13;53(1):71. doi: 10.1186/s13567-022-01089-y. Vet Res. 2022. PMID: 36100945 Free PMC article.
-
The role of Mce proteins in Mycobacterium avium paratuberculosis infection.Sci Rep. 2024 Jun 28;14(1):14964. doi: 10.1038/s41598-024-65592-2. Sci Rep. 2024. PMID: 38942800 Free PMC article.
-
Survey of surface proteins from the pathogenic Mycoplasma hyopneumoniae strain 7448 using a biotin cell surface labeling approach.PLoS One. 2014 Nov 11;9(11):e112596. doi: 10.1371/journal.pone.0112596. eCollection 2014. PLoS One. 2014. PMID: 25386928 Free PMC article.
-
Key role for the alternative sigma factor, SigH, in the intracellular life of Mycobacterium avium subsp. paratuberculosis during macrophage stress.Infect Immun. 2013 Jun;81(6):2242-57. doi: 10.1128/IAI.01273-12. Epub 2013 Apr 8. Infect Immun. 2013. PMID: 23569115 Free PMC article.
-
Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne's Disease.Front Vet Sci. 2017 Nov 6;4:187. doi: 10.3389/fvets.2017.00187. eCollection 2017. Front Vet Sci. 2017. PMID: 29164142 Free PMC article. Review.
References
-
- Alonso, A., and G.-D. Portillo. 2004. Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int. Microbiol. 7181-191. - PubMed
-
- Alto, N. M., F. Shao, C. S. Lazar, R. L. Brost, G. Chua, S. Mattoo, S. A. McMahon, P. Ghosh, T. R. Hughes, C. Boone, and J. E. Dixon. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124133-145. - PubMed
-
- Bannantine, J. P., R. G. Barletta, J. R. Stabel, M. L. Paustian, and V. Kapur. 2004. Application of the genome sequence to address concerns that Mycobacterium avium subspecies paratuberculosis might be a foodborne pathogen. Foodborne Pathog. Dis. 13-15. - PubMed
-
- Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 1492061-2069. - PubMed
-
- Chacon, O., L. E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58329-363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

