A quantitative description of KcsA gating I: macroscopic currents
- PMID: 17938230
- PMCID: PMC2151670
- DOI: 10.1085/jgp.200709843
A quantitative description of KcsA gating I: macroscopic currents
Abstract
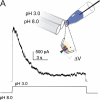
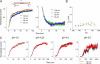
The prokaryotic K(+) channel KcsA is activated by intracellular protons and its gating is modulated by transmembrane voltage. Typically, KcsA functions have been studied under steady-state conditions, using macroscopic Rb(+)-flux experiments and single-channel current measurements. These studies have provided limited insights into the gating kinetics of KcsA due to its low open probability, uncertainties in the number of channels in the patch, and a very strong intrinsic kinetic variability. In this work, we have carried out a detailed analysis of KcsA gating under nonstationary conditions by examining the influence of pH and voltage on the activation, deactivation, and slow-inactivation gating events. We find that activation and deactivation gating of KcsA are predominantly modulated by pH without a significant effect of voltage. Activation gating showed sigmoidal pH dependence with a pKa of approximately 4.2 and a Hill coefficient of approximately 2. In the sustained presence of proton, KcsA undergoes a time-dependent decay of conductance. This inactivation process is pH independent but is modulated by voltage and the nature of permeant ion. Recovery from inactivation occurs via deactivation and also appears to be voltage dependent. We further find that inactivation in KcsA is not entirely a property of the open-conducting channel but can also occur from partially "activated" closed states. The time course of onset and recovery of the inactivation process from these pre-open closed states appears to be different from the open-state inactivation, suggesting the presence of multiple inactivated states with diverse kinetic pathways. This information has been analyzed together with a detailed study of KcsA single-channel behavior (in the accompanying paper) in the framework of a kinetic model. Taken together our data constitutes the first quantitative description of KcsA gating.
Figures
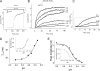
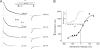






Similar articles
-
A quantitative description of KcsA gating II: single-channel currents.J Gen Physiol. 2007 Nov;130(5):479-96. doi: 10.1085/jgp.200709844. Epub 2007 Oct 15. J Gen Physiol. 2007. PMID: 17938231 Free PMC article.
-
Hysteresis of KcsA potassium channel's activation- deactivation gating is caused by structural changes at the channel's selectivity filter.Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):3234-3239. doi: 10.1073/pnas.1618101114. Epub 2017 Mar 6. Proc Natl Acad Sci U S A. 2017. PMID: 28265056 Free PMC article.
-
Activation-coupled inactivation in the bacterial potassium channel KcsA.Proc Natl Acad Sci U S A. 2005 Dec 6;102(49):17630-5. doi: 10.1073/pnas.0505158102. Epub 2005 Nov 21. Proc Natl Acad Sci U S A. 2005. PMID: 16301524 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Water, proton transfer, and hydrogen bonding in ion channel gating.Front Biosci. 2003 Sep 1;8:s1356-70. doi: 10.2741/1179. Front Biosci. 2003. PMID: 12957850 Review.
Cited by
-
Contact bubble bilayers with flush drainage.Sci Rep. 2015 Mar 16;5:9110. doi: 10.1038/srep09110. Sci Rep. 2015. PMID: 25772819 Free PMC article.
-
Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study.Int J Mol Sci. 2019 Feb 5;20(3):689. doi: 10.3390/ijms20030689. Int J Mol Sci. 2019. PMID: 30764559 Free PMC article.
-
Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration.J Mol Biol. 2010 Aug 13;401(2):155-66. doi: 10.1016/j.jmb.2010.06.031. Epub 2010 Jun 19. J Mol Biol. 2010. PMID: 20600123 Free PMC article.
-
Inactivation in the potassium channel KcsA.J Struct Biol X. 2019 Jun 12;3:100009. doi: 10.1016/j.yjsbx.2019.100009. eCollection 2019 Jul-Sep. J Struct Biol X. 2019. PMID: 32647814 Free PMC article.
-
A quantitative description of KcsA gating II: single-channel currents.J Gen Physiol. 2007 Nov;130(5):479-96. doi: 10.1085/jgp.200709844. Epub 2007 Oct 15. J Gen Physiol. 2007. PMID: 17938231 Free PMC article.
References
-
- Armstrong, C.M. 2003. Voltage-gated K channels. Sci. STKE. 2003:re10. - PubMed
-
- Ayer, R.K., Jr., and F.J. Sigworth. 1997. Enhanced closed-state inactivation in a mutant Shaker K+ channel. J. Membr. Biol. 157:215–230. - PubMed
-
- Baukrowitz, T., and G. Yellen. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 15:951–960. - PubMed
-
- Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. - PubMed

