A quantitative description of KcsA gating II: single-channel currents
- PMID: 17938231
- PMCID: PMC2151667
- DOI: 10.1085/jgp.200709844
A quantitative description of KcsA gating II: single-channel currents
Abstract
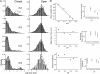
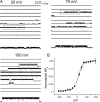
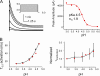
The kinetic transitions of proton-activated WT KcsA and the noninactivating E71A mutant were studied at the single-channel level in purified, liposome-reconstituted preparations. Single-channel currents were recorded using patch-clamp techniques under nonstationary and steady-state conditions. Maximum-likelihood analyses reveal that the key influence of acidic pH is to increase the frequency of bursting without an effect on the intraburst open and closed dwell times, consistent with the finding from macroscopic currents that protons promote activation without a significant effect on inactivation. However, in steady-conditions of pH, voltage not only alters the burst frequency but also affects their properties, such as the frequency of the flickers and the dwell times of the closed and open states. This is to be expected if voltage modulates pathways connecting open and inactivated states. Upon opening, KcsA can enter at least two closed states that are not part of the activation pathway. The frequency and duration of these closed states was found to be voltage dependent and therefore these are likely to represent short-lived inactivated states. Single-channel recordings of WT KcsA also show varying propensity for the presence of subconductance states. The probability of occurrence of these states did not show clear modulation by voltage or pH and their origin remains unclear and a focus for further investigation. A kinetic model is proposed to describe the gating events in KcsA that recapitulates its macroscopic and single-channel behavior. The model has been constrained by the single-channel analyses presented in this work along with data from macroscopic currents in the preceding paper.
Figures









References
-
- Aldrich, R.W., and C.F. Stevens. 1983. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb. Symp. Quant. Biol. 48:147–153. - PubMed
-
- Aldrich, R.W., D.P. Corey, and C.F. Stevens. 1983. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 306:436–441. - PubMed
-
- Ball, F.G., and M.S. Sansom. 1989. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc. R. Soc. Lond. B. Biol. Sci. 236:385–416. - PubMed

