Tarantula toxins interact with voltage sensors within lipid membranes
- PMID: 17938232
- PMCID: PMC2151668
- DOI: 10.1085/jgp.200709869
Tarantula toxins interact with voltage sensors within lipid membranes
Abstract
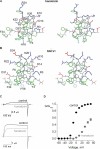
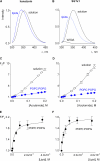
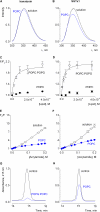
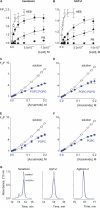
Voltage-activated ion channels are essential for electrical signaling, yet the mechanism of voltage sensing remains under intense investigation. The voltage-sensor paddle is a crucial structural motif in voltage-activated potassium (K(v)) channels that has been proposed to move at the protein-lipid interface in response to changes in membrane voltage. Here we explore whether tarantula toxins like hanatoxin and SGTx1 inhibit K(v) channels by interacting with paddle motifs within the membrane. We find that these toxins can partition into membranes under physiologically relevant conditions, but that the toxin-membrane interaction is not sufficient to inhibit K(v) channels. From mutagenesis studies we identify regions of the toxin involved in binding to the paddle motif, and those important for interacting with membranes. Modification of membranes with sphingomyelinase D dramatically alters the stability of the toxin-channel complex, suggesting that tarantula toxins interact with paddle motifs within the membrane and that they are sensitive detectors of lipid-channel interactions.
Figures




Similar articles
-
Interactions between lipids and voltage sensor paddles detected with tarantula toxins.Nat Struct Mol Biol. 2009 Oct;16(10):1080-5. doi: 10.1038/nsmb.1679. Epub 2009 Sep 27. Nat Struct Mol Biol. 2009. PMID: 19783984 Free PMC article.
-
Molecular Interactions between Tarantula Toxins and Low-Voltage-Activated Calcium Channels.Sci Rep. 2016 Apr 5;6:23894. doi: 10.1038/srep23894. Sci Rep. 2016. PMID: 27045173 Free PMC article.
-
Tarantula toxins use common surfaces for interacting with Kv and ASIC ion channels.Elife. 2015 May 7;4:e06774. doi: 10.7554/eLife.06774. Elife. 2015. PMID: 25948544 Free PMC article.
-
Tarantula toxins interacting with voltage sensors in potassium channels.Toxicon. 2007 Feb;49(2):213-30. doi: 10.1016/j.toxicon.2006.09.024. Epub 2006 Sep 29. Toxicon. 2007. PMID: 17097703 Free PMC article. Review.
-
Tarantulas: eight-legged pharmacists and combinatorial chemists.Toxicon. 2004 Apr;43(5):555-74. doi: 10.1016/j.toxicon.2004.02.007. Toxicon. 2004. PMID: 15066413 Review.
Cited by
-
Exploring structure-function relationships between TRP and Kv channels.Sci Rep. 2013;3:1523. doi: 10.1038/srep01523. Sci Rep. 2013. PMID: 23519328 Free PMC article.
-
Solution structure of kurtoxin: a gating modifier selective for Cav3 voltage-gated Ca(2+) channels.Biochemistry. 2012 Mar 6;51(9):1862-73. doi: 10.1021/bi201633j. Epub 2012 Feb 22. Biochemistry. 2012. PMID: 22329781 Free PMC article.
-
Voltage-gated channel mechanosensitivity: fact or friction?Front Physiol. 2011 May 31;2:25. doi: 10.3389/fphys.2011.00025. eCollection 2011. Front Physiol. 2011. PMID: 21660289 Free PMC article.
-
The hitchhiker's guide to the voltage-gated sodium channel galaxy.J Gen Physiol. 2016 Jan;147(1):1-24. doi: 10.1085/jgp.201511492. J Gen Physiol. 2016. PMID: 26712848 Free PMC article. Review.
-
Opening the shaker K+ channel with hanatoxin.J Gen Physiol. 2013 Feb;141(2):203-16. doi: 10.1085/jgp.201210914. J Gen Physiol. 2013. PMID: 23359283 Free PMC article.
References
-
- Ahern, C.A., and R. Horn. 2004. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 27:303–307. - PubMed
-
- Andersen, O.S., C. Nielsen, A.M. Maer, J.A. Lundbaek, M. Goulian, and R.E. Koeppe II. 1999. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 294:208–224. - PubMed
-
- Bemporad, D., Z.A. Sands, C.L. Wee, A. Grottesi, and M.S. Sansom. 2006. Vstx1, a modifier of Kv channel gating, localizes to the interfacial region of lipid bilayers. Biochemistry. 45:11844–11855. - PubMed
-
- Beschiaschvili, G., and J. Seelig. 1990. Melittin binding to mixed phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 29:52–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

