Identification of an alternative G{alpha}q-dependent chemokine receptor signal transduction pathway in dendritic cells and granulocytes
- PMID: 17938235
- PMCID: PMC2118484
- DOI: 10.1084/jem.20071267
Identification of an alternative G{alpha}q-dependent chemokine receptor signal transduction pathway in dendritic cells and granulocytes
Abstract
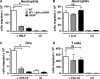
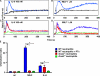
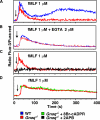
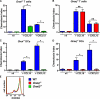
CD38 controls the chemotaxis of leukocytes to some, but not all, chemokines, suggesting that chemokine receptor signaling in leukocytes is more diverse than previously appreciated. To determine the basis for this signaling heterogeneity, we examined the chemokine receptors that signal in a CD38-dependent manner and identified a novel "alternative" chemokine receptor signaling pathway. Similar to the "classical" signaling pathway, the alternative chemokine receptor pathway is activated by Galpha(i2)-containing Gi proteins. However, unlike the classical pathway, the alternative pathway is also dependent on the Gq class of G proteins. We show that Galpha(q)-deficient neutrophils and dendritic cells (DCs) make defective calcium and chemotactic responses upon stimulation with N-formyl methionyl leucyl phenylalanine and CC chemokine ligand (CCL) 3 (neutrophils), or upon stimulation with CCL2, CCL19, CCL21, and CXC chemokine ligand (CXCL) 12 (DCs). In contrast, Galpha(q)-deficient T cell responses to CXCL12 and CCL19 remain intact. Thus, the alternative chemokine receptor pathway controls the migration of only a subset of cells. Regardless, the novel alternative chemokine receptor signaling pathway appears to be critically important for the initiation of inflammatory responses, as Galpha(q) is required for the migration of DCs from the skin to draining lymph nodes after fluorescein isothiocyanate sensitization and the emigration of monocytes from the bone marrow into inflamed skin after contact sensitization.
Figures


Similar articles
-
Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling.J Immunol. 2004 May 1;172(9):5175-84. doi: 10.4049/jimmunol.172.9.5175. J Immunol. 2004. PMID: 15100254
-
Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity.Immunity. 2004 Mar;20(3):279-91. doi: 10.1016/s1074-7613(04)00048-2. Immunity. 2004. PMID: 15030772
-
Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells.Immunobiology. 2014 Mar;219(3):218-29. doi: 10.1016/j.imbio.2013.10.004. Epub 2013 Oct 14. Immunobiology. 2014. PMID: 24268109
-
The multiple personalities of the chemokine receptor CCR7 in dendritic cells.J Immunol. 2006 May 1;176(9):5153-9. doi: 10.4049/jimmunol.176.9.5153. J Immunol. 2006. PMID: 16621978 Review.
-
Leukocyte migration: scent of the T zone.Curr Biol. 2000 Jan 13;10(1):R30-3. doi: 10.1016/s0960-9822(99)00253-5. Curr Biol. 2000. PMID: 10660291 Review.
Cited by
-
Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling.J Clin Invest. 2008 Mar;118(3):1074-84. doi: 10.1172/JCI33187. J Clin Invest. 2008. PMID: 18274673 Free PMC article.
-
G alpha q-containing G proteins regulate B cell selection and survival and are required to prevent B cell-dependent autoimmunity.J Exp Med. 2010 Aug 2;207(8):1775-89. doi: 10.1084/jem.20092735. Epub 2010 Jul 12. J Exp Med. 2010. PMID: 20624888 Free PMC article.
-
MCP-1/CCR2B-dependent loop upregulates MUC5AC and MUC5B in human airway epithelium.Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L204-15. doi: 10.1152/ajplung.00292.2010. Epub 2010 Nov 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21097527 Free PMC article.
-
Role of G protein-coupled receptors in inflammation.Acta Pharmacol Sin. 2012 Mar;33(3):342-50. doi: 10.1038/aps.2011.200. Epub 2012 Feb 27. Acta Pharmacol Sin. 2012. PMID: 22367283 Free PMC article. Review.
-
Heterozygous Gnaq deficiency enhances Ifi202b/IFI16 and NF-κB activation in endothelial cells and exacerbates lupus nephritis pathology.iScience. 2024 Jun 22;27(8):110350. doi: 10.1016/j.isci.2024.110350. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108722 Free PMC article.
References
-
- Wettschureck, N., and S. Offermanns. 2005. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85:1159–1204. - PubMed
-
- Polakis, P.G., R.J. Uhing, and R. Snyderman. 1988. The formylpeptide chemoattractant receptor copurifies with a GTP-binding protein containing a distinct 40-kDa pertussis toxin substrate. J. Biol. Chem. 263:4969–4976. - PubMed
-
- Ye, R.D., O. Quehenberger, K.M. Thomas, J. Navarro, S.L. Cavanagh, E.R. Prossnitz, and C.G. Cochrane. 1993. The rabbit neutrophil N-formyl peptide receptor. cDNA cloning, expression, and structure/function implications. J. Immunol. 150:1383–1394. - PubMed
-
- Schreiber, R.E., E.R. Prossnitz, R.D. Ye, C.G. Cochrane, A.J. Jesaitis, and G.M. Bokoch. 1993. Reconstitution of recombinant N-formyl chemotactic peptide receptor with G protein. J. Leukoc. Biol. 53:470–474. - PubMed
-
- Damaj, B.B., S.R. McColl, W. Mahana, M.F. Crouch, and P.H. Naccache. 1996. Physical association of Gi2alpha with interleukin-8 receptors. J. Biol. Chem. 271:12783–12789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

