DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys?
- PMID: 17938249
- PMCID: PMC2064754
- DOI: 10.1083/jcb.200705106
DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys?
Abstract
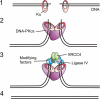
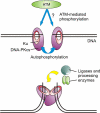
The DNA-dependent protein kinase (DNA-PK) is one of the central enzymes involved in DNA double-strand break (DSB) repair. It facilitates proper alignment of the two ends of the broken DNA molecule and coordinates access of other factors to the repair complex. We discuss the latest findings on DNA-PK phosphorylation and offer a working model for the regulation of DNA-PK during DSB repair.
Figures

Similar articles
-
Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks.J Cell Biol. 2007 Apr 23;177(2):219-29. doi: 10.1083/jcb.200608077. Epub 2007 Apr 16. J Cell Biol. 2007. PMID: 17438073 Free PMC article.
-
DNA-PK: a dynamic enzyme in a versatile DSB repair pathway.DNA Repair (Amst). 2014 May;17:21-9. doi: 10.1016/j.dnarep.2014.02.020. Epub 2014 Mar 27. DNA Repair (Amst). 2014. PMID: 24680878 Free PMC article. Review.
-
DNA-PK: the means to justify the ends?Adv Immunol. 2008;99:33-58. doi: 10.1016/S0065-2776(08)00602-0. Adv Immunol. 2008. PMID: 19117531 Review.
-
trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining.Mol Cell Biol. 2007 May;27(10):3881-90. doi: 10.1128/MCB.02366-06. Epub 2007 Mar 12. Mol Cell Biol. 2007. PMID: 17353268 Free PMC article.
-
Inhibition of homologous recombination by DNA-dependent protein kinase requires kinase activity, is titratable, and is modulated by autophosphorylation.Mol Cell Biol. 2011 Apr;31(8):1719-33. doi: 10.1128/MCB.01298-10. Epub 2011 Feb 7. Mol Cell Biol. 2011. PMID: 21300785 Free PMC article.
Cited by
-
Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci.J Biol Chem. 2012 Mar 16;287(12):8803-15. doi: 10.1074/jbc.M111.320887. Epub 2012 Jan 23. J Biol Chem. 2012. PMID: 22270370 Free PMC article.
-
Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer.Cancer Cell. 2011 May 17;19(5):664-78. doi: 10.1016/j.ccr.2011.04.010. Cancer Cell. 2011. PMID: 21575865 Free PMC article.
-
DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks.Nat Struct Mol Biol. 2012 Feb 12;19(3):276-82. doi: 10.1038/nsmb.2224. Nat Struct Mol Biol. 2012. PMID: 22343725
-
Dephosphorylation of γ-H2AX by WIP1: an important homeostatic regulatory event in DNA repair and cell cycle control.Cell Cycle. 2010 Jun 1;9(11):2092-6. doi: 10.4161/cc.9.11.11810. Epub 2010 Jun 1. Cell Cycle. 2010. PMID: 20495376 Free PMC article.
-
DNA-PK target identification reveals novel links between DNA repair signaling and cytoskeletal regulation.PLoS One. 2013 Nov 25;8(11):e80313. doi: 10.1371/journal.pone.0080313. eCollection 2013. PLoS One. 2013. PMID: 24282534 Free PMC article.
References
-
- Ahnesorg, P., P. Smith, and S.P. Jackson. 2006. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 124:301–313. - PubMed
-
- Burma, S., and D.J. Chen. 2004. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst.). 3:909–918. - PubMed
-
- Chen, B.P., D.W. Chan, J. Kobayashi, S. Burma, A. Asaithamby, K. Morotomi-Yano, E. Botvinick, J. Qin, and D.J. Chen. 2005. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J. Biol. Chem. 280:14709–14715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

