Side-chain protonation and mobility in the sarcoplasmic reticulum Ca2+-ATPase: implications for proton countertransport and Ca2+ release
- PMID: 17938423
- PMCID: PMC2025671
- DOI: 10.1529/biophysj.107.109363
Side-chain protonation and mobility in the sarcoplasmic reticulum Ca2+-ATPase: implications for proton countertransport and Ca2+ release
Abstract
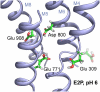
Protonation of acidic residues in the sarcoplasmic reticulum Ca(2+)-ATPase (SERCA 1a) was studied by multiconformation continuum electrostatic calculations in the Ca(2+)-bound state Ca(2)E1, in the Ca(2+)-free state E2(TG) with bound thapsigargin, and in the E2P (ADP-insensitive phosphoenzyme) analog state with MgF(4)(2-) E2(TG+MgF(4)(2-)). Around physiological pH, all acidic Ca(2+) ligands (Glu(309), Glu(771), Asp(800), and Glu(908)) were unprotonated in Ca(2)E1; in E2(TG) and E2(TG+MgF(4)(2-)) Glu(771), Asp(800), and Glu(908) were protonated. Glu(771) and Glu(908) had calculated pK(a) values larger than 14 in E2(TG) and E2(TG+MgF(4)(2-)), whereas Asp(800) titrated with calculated pK(a) values near 7.5. Glu(309) had very different pK(a) values in the Ca(2+)-free states: 8.4 in E2(TG+MgF(4)(2-)) and 4.7 in E2(TG) because of a different local backbone conformation. This indicates that Glu(309) can switch between a high and a low pK(a) mode, depending on the local backbone conformation. Protonated Glu(309) occupied predominantly two main, very differently orientated side-chain conformations in E2(TG+MgF(4)(2-)): one oriented inward toward the other Ca(2+) ligands and one oriented outward toward a protein channel that seems to be in contact with the cytoplasm. Upon deprotonation, Glu(309) adopted completely the outwardly orientated side-chain conformation. The contact of Glu(309) with the cytoplasm in E2(TG+MgF(4)(2-)) makes this residue unlikely to bind lumenal protons. Instead it might serve as a proton shuttle between Ca(2+)-binding site I and the cytoplasm. Glu(771), Asp(800), and Glu(908) are proposed to take part in proton countertransport.
Figures

Similar articles
-
Protonation and hydrogen bonding of Ca2+ site residues in the E2P phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase studied by a combination of infrared spectroscopy and electrostatic calculations.Biophys J. 2008 Jan 15;94(2):600-11. doi: 10.1529/biophysj.107.114033. Epub 2007 Sep 21. Biophys J. 2008. PMID: 17890386 Free PMC article.
-
Distinct natures of beryllium fluoride-bound, aluminum fluoride-bound, and magnesium fluoride-bound stable analogues of an ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase: changes in catalytic and transport sites during phosphoenzyme hydrolysis.J Biol Chem. 2004 Apr 9;279(15):14991-8. doi: 10.1074/jbc.M313363200. Epub 2004 Jan 30. J Biol Chem. 2004. PMID: 14754887
-
Proton paths in the sarcoplasmic reticulum Ca(2+) -ATPase.Biochim Biophys Acta. 2007 Nov;1767(11):1310-8. doi: 10.1016/j.bbabio.2007.07.010. Epub 2007 Aug 16. Biochim Biophys Acta. 2007. PMID: 17904096
-
Ion transport and energy transduction of P-type ATPases: implications from electrostatic calculations.Biochim Biophys Acta. 2009 Jun;1787(6):721-9. doi: 10.1016/j.bbabio.2009.02.015. Epub 2009 Mar 2. Biochim Biophys Acta. 2009. PMID: 19265666 Review.
-
Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase.J Biol Chem. 2013 Apr 12;288(15):10759-65. doi: 10.1074/jbc.R112.436550. Epub 2013 Feb 11. J Biol Chem. 2013. PMID: 23400778 Free PMC article. Review.
Cited by
-
Protonation and hydrogen bonding of Ca2+ site residues in the E2P phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase studied by a combination of infrared spectroscopy and electrostatic calculations.Biophys J. 2008 Jan 15;94(2):600-11. doi: 10.1529/biophysj.107.114033. Epub 2007 Sep 21. Biophys J. 2008. PMID: 17890386 Free PMC article.
-
Dipole-Potential-Mediated Effects on Ion Pump Kinetics.Biophys J. 2015 Oct 20;109(8):1513-20. doi: 10.1016/j.bpj.2015.08.022. Biophys J. 2015. PMID: 26488640 Free PMC article. Review.
-
High-yield heterologous expression of wild type and mutant Ca(2+) ATPase: Characterization of Ca(2+) binding sites by charge transfer.J Mol Biol. 2009 Sep 4;391(5):858-71. doi: 10.1016/j.jmb.2009.06.044. Epub 2009 Jun 24. J Mol Biol. 2009. PMID: 19559032 Free PMC article.
-
Proton Countertransport and Coupled Gating in the Sarcoplasmic Reticulum Calcium Pump.J Mol Biol. 2018 Dec 7;430(24):5050-5065. doi: 10.1016/j.jmb.2018.10.014. Epub 2018 Oct 26. J Mol Biol. 2018. PMID: 30539761 Free PMC article.
-
Atomic-level characterization of the activation mechanism of SERCA by calcium.PLoS One. 2011;6(10):e26936. doi: 10.1371/journal.pone.0026936. Epub 2011 Oct 27. PLoS One. 2011. PMID: 22046418 Free PMC article.
References
-
- Hasselbach, W., and M. Makinose. 1961. The calcium pump of the relaxing granules of muscle and its dependence on ATP splitting. Biochem. Z. 333:518–528. - PubMed
-
- Mintz, E., and F. Guillain. 1997. Ca2+ transport by the sarcoplasmic reticulum ATPase. Biochim. Biophys. Acta. 1318:52–70. - PubMed
-
- Toyoshima, C., and G. Inesi. 2004. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 73:269–292. - PubMed
-
- Stokes, D. L., and N. M. Green. 2003. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. 32:445–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

