Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell
- PMID: 17939748
- PMCID: PMC3197825
- DOI: 10.1089/hum.2007.090
Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell
Abstract
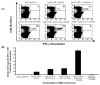
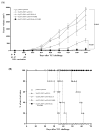
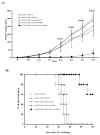
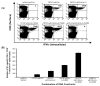
Intradermal administration of DNA vaccines, using a gene gun, represents an effective means of delivering DNA directly into professional antigen-presenting cells (APCs) in the skin and thus allows the application of strategies to modify the properties of APCs to enhance DNA vaccine potency. In the current study, we hypothesized that the potency of human papillomavirus (HPV) type 16 E7 DNA vaccines employing intracellular targeting strategies combined with a strategy to prolong the life of dendritic cells (DCs) could be further enhanced by the addition of a DNA vaccine capable of generating high numbers of pan-HLA-DR reactive epitope (PADRE)-specific CD4(+) T cells. We observed that the addition of PADRE DNA to E7 DNA vaccines employing intracellular targeting strategies with a strategy to prolong the life of DCs led to significant enhancement of E7-specific CD8(+) effector and memory T cells as well as significantly improved therapeutic effects against established E7-expressing tumors in tumor-challenged mice. Our data suggest that the potency of a DNA vaccine combining an intracellular targeting strategy as well as a strategy to prolong the life of DCs can be further enhanced by addition of DNA that is capable of generating high numbers of PADRE-specific CD4(+) T cells.
Conflict of interest statement
For all authors, no competing financial interests exist.
Figures

Similar articles
-
Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun.J Immunol. 2008 May 15;180(10):7019-27. doi: 10.4049/jimmunol.180.10.7019. J Immunol. 2008. PMID: 18453624 Free PMC article.
-
Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies.J Immunol. 2003 Sep 15;171(6):2970-6. doi: 10.4049/jimmunol.171.6.2970. J Immunol. 2003. PMID: 12960321
-
CD4+ TH1 cells generated by Ii-PADRE DNA at prime phase are important to induce effectors and memory CD8+ T cells.J Immunother. 2010 Jun;33(5):510-22. doi: 10.1097/CJI.0b013e3181d75cef. J Immunother. 2010. PMID: 20463596
-
Modifying professional antigen-presenting cells to enhance DNA vaccine potency.Methods Mol Med. 2006;127:199-220. doi: 10.1385/1-59745-168-1:199. Methods Mol Med. 2006. PMID: 16988456 Review.
-
Technologies for enhanced efficacy of DNA vaccines.Expert Rev Vaccines. 2012 Feb;11(2):189-209. doi: 10.1586/erv.11.188. Expert Rev Vaccines. 2012. PMID: 22309668 Free PMC article. Review.
Cited by
-
Improvement of cytomegalovirus pp65 DNA vaccine efficacy by co-administration of siRNAs targeting BAK and BAX.Exp Ther Med. 2017 Jun;13(6):3275-3280. doi: 10.3892/etm.2017.4385. Epub 2017 Apr 26. Exp Ther Med. 2017. PMID: 28587400 Free PMC article.
-
Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun.J Immunol. 2008 May 15;180(10):7019-27. doi: 10.4049/jimmunol.180.10.7019. J Immunol. 2008. PMID: 18453624 Free PMC article.
-
DNA vaccines for cervical cancer: from bench to bedside.Exp Mol Med. 2007 Dec 31;39(6):679-89. doi: 10.1038/emm.2007.74. Exp Mol Med. 2007. PMID: 18160838 Free PMC article. Review.
-
Therapeutic HPV DNA vaccines.Immunol Res. 2010 Jul;47(1-3):86-112. doi: 10.1007/s12026-009-8141-6. Immunol Res. 2010. PMID: 20066511 Free PMC article. Review.
-
Promoting effect of Antrodia camphorata as an immunomodulating adjuvant on the antitumor efficacy of HER-2/neu DNA vaccine.Cancer Immunol Immunother. 2010 Aug;59(8):1259-72. doi: 10.1007/s00262-010-0852-y. Cancer Immunol Immunother. 2010. PMID: 20390417 Free PMC article.
References
-
- BEVAN MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. - PubMed
-
- BOUSSO P, ROBEY E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. - PubMed
-
- CASTELLINO F, GERMAIN RN. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu Rev Immunol. 2006;24:519–540. - PubMed
-
- CASTELLINO F, HUANG AY, ALTAN-BONNET G, STOLL S, SCHEINECKER C, GERMAIN RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature. 2006;440:890–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

