EGF receptor ubiquitination is not necessary for its internalization
- PMID: 17940017
- PMCID: PMC2040475
- DOI: 10.1073/pnas.0707416104
EGF receptor ubiquitination is not necessary for its internalization
Erratum in
- Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14180
Abstract
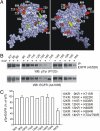
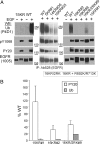
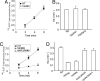
Ubiquitination of the EGF receptor (EGFR) has been implicated in EGF-induced receptor internalization, lysosomal degradation, and down-regulation. Mutation of EGFR ubiquitination sites identified by mass spectrometry yielded receptor mutants that are weakly ubiquitinated and not down-regulated by EGF. However, these EGFR mutants were normally internalized. To examine whether this internalization was mediated by the residual ubiquitination, systematic mutagenesis of lysine residues in the kinase domain of the EGFR was performed to generate a receptor mutant that is not ubiquitinated. Mutations of a number of lysines inhibited kinase activity of the EGFR, thus leading to the inhibition of receptor internalization. However, a mutant lacking 15 lysine residues (15KR), which was negligibly ubiquitinated and normally phosphorylated, was internalized at a rate similar to that of the wild-type EGFR. As in the case of the wild-type EGFR, internalization of the 15KR mutant depended on the presence of clathrin, Grb2 adaptor, and Cbl ubiquitin ligase. These data imply that EGFR ubiquitination is not necessary for its internalization by clathrin-coated pits. Interestingly, the reconstitution of two major ubiquitination sites in the 16KR receptor mutant, which had impaired kinase activity and slow internalization kinetics, resulted in a partial rescue of ubiquitination and a complete rescue of receptor internalization. This result suggests that ubiquitination of the kinase-impaired receptor can mediate its internalization by the clathrin pathway. Altogether these data emphasize the robustness of the EGFR internalization process, which can be controlled by multiple kinase- and ubiquitination-dependent and -independent mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
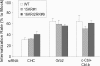

References
-
- Schlessinger J. Cell. 2000;103:211–225. - PubMed
-
- Sorkin A, Von Zastrow M. Nat Rev Mol Cell Biol. 2002;3:600–614. - PubMed
-
- Di Fiore PP, Gill GN. Curr Opin Cell Biol. 1999;11:483–488. - PubMed
-
- Marmor MD, Yarden Y. Oncogene. 2004;23:2057–2070. - PubMed
-
- Miranda M, Sorkin A. Mol Interv. 2007;7:157–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

