Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis
- PMID: 17940034
- PMCID: PMC2040463
- DOI: 10.1073/pnas.0704680104
Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis
Abstract
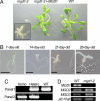
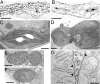
The biogenesis of thylakoid membranes, an indispensable event for the photoautotrophic growth of plants, requires a significant increase in the level of the unique thylakoid membrane lipid monogalactosyldiacylglycerol (MGDG), which constitutes the bulk of membrane lipids in chloroplasts. The final step in MGDG biosynthesis occurs in the plastid envelope and is catalyzed by MGDG synthase. Here we report the identification and characterization of an Arabidopsis mutant showing a complete defect in MGDG synthase 1. The mutant seeds germinated as small albinos only in the presence of sucrose. The seedlings lacked galactolipids and had disrupted photosynthetic membranes, leading to the complete impairment of photosynthetic ability and photoautotrophic growth. Moreover, invagination of the inner envelope, which is not seen in mature WT chloroplasts, was observed in the mutant, supporting an old hypothesis that envelope invagination is a major event in early chloroplast biogenesis. In addition to the defective seedling phenotype, embryo development was arrested in the mutant, although seeds with impaired embryos could germinate heterotrophically. These results demonstrate the importance of galactolipids not only in photosynthetic growth but also in embryogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Block MA, Dorne AJ, Joyard J, Douce R. J Biol Chem. 1983;258:13281–13286. - PubMed
-
- Dörmann P, Benning C. Trends Plant Sci. 2002;7:112–118. - PubMed
-
- Lee AG. Curr Biol. 2000;10:R377–R380. - PubMed
-
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Nature. 2001;411:909–917. - PubMed
-
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Nature. 2005;438:1040–1044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

