HOXB4's road map to stem cell expansion
- PMID: 17940039
- PMCID: PMC2040480
- DOI: 10.1073/pnas.0703082104
HOXB4's road map to stem cell expansion
Abstract
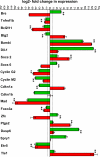
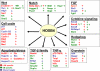
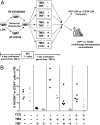
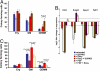
Homeodomain-containing transcription factors are important regulators of stem cell behavior. HOXB4 mediates expansion of adult and embryo-derived hematopoietic stem cells (HSCs) when expressed ectopically. To define the underlying molecular mechanisms, we performed gene expression profiling in combination with subsequent functional analysis with enriched adult HSCs and embryonic derivatives expressing inducible HOXB4. Thereby, we identified a set of overlapping genes that likely represent "universal" targets of HOXB4. A substantial number of loci are involved in signaling pathways important for controlling self-renewal, maintenance, and differentiation of stem cells. Functional assays performed on selected pathways confirmed the biological coherence of the array results. HOXB4 activity protected adult HSCs from the detrimental effects mediated by the proinflammatory cytokine TNF-alpha. This protection likely contributes to the competitive repopulation advantage of HOXB4-expressing HSCs observed in vivo. The concept of TNF-alpha inhibition may also prove beneficial for patients undergoing bone marrow transplantation. Furthermore, we demonstrate that HOXB4 activity and FGF signaling are intertwined. HOXB4-mediated expansion of adult and ES cell-derived HSCs was enhanced by specific and complete inhibition of FGF receptors. In contrast, the expanding activity of HOXB4 on hematopoietic progenitors in day 4-6 embryoid bodies was blunted in the presence of basic FGF (FGF2), indicating a dominant negative effect of FGF signaling on the earliest hematopoietic cells. In summary, our results strongly suggest that HOXB4 modulates the response of HSCs to multiple extrinsic signals in a concerted manner, thereby shifting the balance toward stem cell self-renewal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Akala O, Clark MF. Curr Opin Genet Dev. 2006;16:1–6. - PubMed
-
- Antonchuck J, Sauvageau G, Humphries RK. Cell. 2002;10:39–45. - PubMed
-
- Antonchuck J, Sauvageau G, Humphries RK. Exp Hematol. 2001;29:1125–1134. - PubMed
-
- Kyba M, Perlingeiro RC, Daley GQ. Cell. 2002;109:29–37. - PubMed
-
- Rideout WM, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Cell. 2002;109:17–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

