Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery
- PMID: 17940116
- PMCID: PMC2190749
- DOI: 10.1210/jc.2007-1538
Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery
Abstract
Context: Chorioamnionitis (CAM)-elicited preterm delivery (PTD) is associated with elevated amniotic fluid levels of IL-1beta and TNF-alpha. We hypothesized that IL-1beta and TNF-alpha may induce matrix metalloproteinase (MMP)-1 and MMP-3 activity to promote PTD by degrading decidual and fetal membranes and cervical extracellular matrix.
Objective: Our objective was to evaluate: 1) MMP-1 and MMP-3 expression in decidual sections from uncomplicated term, idiopathic preterm, and CAM-complicated deliveries, and 2) the separate and interactive effects of IL-1beta, TNF-alpha, medroxyprogesterone acetate (MPA), and a p38 MAPK inhibitor (SB203580) on MMP-1 and MMP-3 expression in term decidual cells (DCs).
Interventions and main outcome measures: Decidua were immunostained for MMP-1 and MMP-3. Cultured term DCs were incubated with estradiol (E2) or E2 plus MPA with or without IL-1beta or TNF-alpha with or without SB203580. ELISA and Western blotting assessed secreted MMP-1 and MMP-3 levels, quantitative real-time RT-PCR assessed mRNA levels, and substrate gel zymography was used to determined MMP-1 and MMP-3 proteolytic activity.
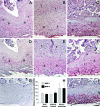
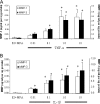
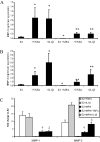
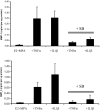
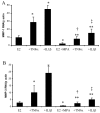
Results: MMP-1 and MMP-3 immunostaining was more prominent in CAM-complicated decidua vs. control preterm and term decidual specimens (P < 0.05). Compared with basal outputs by DCs incubated with E2, TNF-alpha enhanced MMP-1 and MMP-3 secretion by 14 +/- 3- and 9 +/- 2-fold, respectively, and IL-1beta increased MMP-1 and MMP-3 secretion by 13 +/- 3- and 19 +/- 2-fold, respectively (P < 0.05). Addition of MPA lowered basal MMP-1 and MMP-3 outputs by 70%, whereas the TNF-alpha- and IL-1beta-enhanced MMP-1 and MMP-3 levels were blunted by more than 50% (P < 0.05). SB203580 suppressed TNF-alpha- and IL-1beta-induced MMP-1 and MMP-3 secretion by severalfold. Western blotting confirmed the ELISA results, and mRNA levels corresponded with MMP-1 and MMP-3 protein levels. MMP-1 and MMP-3 proteolytic activity was confirmed by substrate gel zymography.
Conclusion: Augmented DC-expressed MMP-1 and MMP-3 in CAM-complicated pregnancies may promote PTD via decidual, fetal membrane, and cervical extracellular matrix degradation. Effects of progestin-p38 MAPK signaling inhibition on cytokine-enhanced MMP-1 and MMP-3 expression in term DCs suggest alternative mechanisms to prevent CAM-induced PTD.
Figures

Similar articles
-
Progestin suppresses thrombin- and interleukin-1beta-induced interleukin-11 production in term decidual cells: implications for preterm delivery.J Clin Endocrinol Metab. 2005 Sep;90(9):5279-86. doi: 10.1210/jc.2005-0210. Epub 2005 Jul 5. J Clin Endocrinol Metab. 2005. PMID: 15998775
-
Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis.Am J Pathol. 2006 Oct;169(4):1294-302. doi: 10.2353/ajpath.2006.060185. Am J Pathol. 2006. PMID: 17003486 Free PMC article.
-
Progestin and thrombin regulate tissue factor expression in human term decidual cells.J Clin Endocrinol Metab. 2009 Jun;94(6):2164-70. doi: 10.1210/jc.2009-0065. Epub 2009 Mar 10. J Clin Endocrinol Metab. 2009. PMID: 19276228 Free PMC article.
-
Regulation of interleukin-6 expression in human decidual cells and its potential role in chorioamnionitis.Am J Pathol. 2010 Oct;177(4):1755-64. doi: 10.2353/ajpath.2010.090781. Epub 2010 Aug 19. Am J Pathol. 2010. PMID: 20724602 Free PMC article.
-
The matrix metalloproteinases (MMPS) in the decidua and fetal membranes.Front Biosci. 2007 Jan 1;12:649-59. doi: 10.2741/2089. Front Biosci. 2007. PMID: 17127325 Review.
Cited by
-
Escherichia coli induced matrix metalloproteinase-9 activity and type IV collagen degradation is regulated by progesterone in human maternal decidual.BMC Pregnancy Childbirth. 2024 Oct 4;24(1):645. doi: 10.1186/s12884-024-06847-8. BMC Pregnancy Childbirth. 2024. PMID: 39367340 Free PMC article.
-
PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV.Mol Endocrinol. 2013 Jul;27(7):1048-64. doi: 10.1210/me.2012-1322. Epub 2013 Jun 6. Mol Endocrinol. 2013. PMID: 23744893 Free PMC article.
-
Effects of histone H4 hyperacetylation on inhibiting MMP2 and MMP9 in human amniotic epithelial cells and in premature rupture of fetal membranes.Exp Ther Med. 2021 May;21(5):515. doi: 10.3892/etm.2021.9946. Epub 2021 Mar 22. Exp Ther Med. 2021. PMID: 33815588 Free PMC article.
-
Preeclampsia-related increase of interleukin-11 expression in human decidual cells.Reproduction. 2010 Oct;140(4):605-12. doi: 10.1530/REP-10-0263. Epub 2010 Jul 28. Reproduction. 2010. PMID: 20668109 Free PMC article.
-
The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding.Hum Reprod Update. 2016 Jun;22(4):497-515. doi: 10.1093/humupd/dmw004. Epub 2016 Feb 23. Hum Reprod Update. 2016. PMID: 26912000 Free PMC article. Review.
References
-
- Mueller-Heubach E, Rubinstein DN, Schwarz SS 1990 Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol 75:622–626 - PubMed
-
- Lockwood CJ, Kuczynski E 1999 Markers of risk for preterm delivery. J Perinat Med 27:5–20 - PubMed
-
- Hamilton BE, Martin JA, Ventura SJ 2006 Births: preliminary data for 2005. Natl Vital Stat Rep 55:1–18 - PubMed
-
- Newton ER 2005 Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 32:571–600 - PubMed
-
- Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH 2004 Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 191:1339–1345 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

